InnoCare Pharma Limited (“InnoCare or “InnoCare Pharma”) (HKEX: 09969; SSE: 688428) and Zenas BioPharma, Inc. (“Zenas,”“Zenas BioPharma” or the “Company”) (Nasdaq: ZBIO) announced a transformational license agreement granting Zenas global development and commercialization rights to orelabrutinib for Multiple Sclerosis (MS) and across all therapeutic areas other than oncology. Zenas also secured rights to a novel oral IL-17AA/AF inhibitor, and an oral, brain-penetrant, TYK2 inhibitor.
Orelabrutinib is a potentially best-in-class, highly CNS-penetrant and selective, oral small molecule BTK inhibitor with the potential to address compartmentalized inflammation and disease progression in MS. A global, Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial evaluating the safety and efficacy of orelabrutinib dosed 80 mg once daily (QD) in patients with Primary Progressive MS (PPMS) has been initiated. Zenas plans to initiate a second global, Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial evaluating orelabrutinib in patients with Secondary Progressive MS (SPMS) in the first quarter of 2026.
In a previously completed global Phase 2 clinical trial in patients with Relapsing-Remitting MS (RRMS), orelabrutinib demonstrated significant reductions in new Gd+ T1 lesions versus placebo at weeks 12 and 24, with sustained reductions in inflammatory activity through week 96 as demonstrated by meaningful impact on endpoints indicative of disease progression. The safety and tolerability profile of orelabrutinib is consistent with other BTK inhibitors in development for MS and is well characterized across multiple prior autoimmune disease and hematologic cancer trials.
“We are delighted to partner with Zenas BioPharma. The partnership with Zenas BioPharma represents a significant milestone in our journey, and we will continue to enhance and advance our globalization efforts in the future,” said Dr. Jasmine Cui, Co-Founder, Chairwoman and CEO of InnoCare Pharma. “Orelabrutinib has a differentiated mechanism of action and strong clinical data underscoring its promising potential as a treatment for patients with progressive forms of MS. We are confident in Zenas’ management team given their exceptional track record of successful drug development, global regulatory approvals and commercial launches, and their commitment to driving innovation for autoimmune diseases.”
“InnoCare is a globally recognized company with a successful track record of drug discovery, development and commercialization. This transformative collaboration with InnoCare further positions Zenas to execute on its vision to become a global, fully integrated development and commercial-stage autoimmune-focused biopharmaceutical company. With global rights to orelabrutinib, we are advancing a potential blockbuster franchise for progressive MS. Orelabrutinib, with its best-in-class potential, is strongly positioned to address disease progression independent of relapse activity, the highest unmet medical need in MS, and to improve the lives of patients living with progressive MS. We are also excited to add two potentially best-in-class molecules, a novel oral, IL-17AA/AF inhibitor and an oral, brain-penetrant, TYK2 inhibitor, to our pipeline. We plan to advance each of these programs to human clinical trials in 2026 and expect to have initial patient data from the oral IL-17AA/AF clinical program in 2027,” said Lonnie Moulder, Founder and Chief Executive Officer (CEO) of Zenas.
“This strategic collaboration will leverage our shared focus to accelerate the development of orelabrutinib and help maximize its clinical and commercial potential on a global scale, particularly in MS,” added Dr. Cui, “Given the statistically significant and clinically meaningful data from the Phase 2 trial, and promising blood-brain barrier penetration capability, orelabrutinib has the potential to transform the treatment paradigm for this devastating disease. In addition to orelabrutinib, we have fortified our powerful discovery engine to focus on cutting-edge targets for the development of autoimmune therapeutics through B-cell and T-cell pathways, with the aim of delivering first-in-class and/or best-in-class treatments to address the massive unmet medical needs and strong market potential in China and worldwide.”
Mr. Moulder continued, “With this transaction we have established a balanced portfolio of complementary mechanisms and modalities with best-in-class blockbuster potential across multiple therapeutic areas. With our two franchise programs, obexelimab concluding Phase 3 development for IgG4-RD, and now orelabrutinib for progressive forms of MS, Zenas is well positioned to meaningfully impact the lives of patients living with autoimmune diseases. We are prioritizing the tremendous opportunity ahead with orelabrutinib in PPMS and SPMS. We expect to report obexelimab topline 12-week primary endpoint results from the Phase 2 MoonStone trial in patients with RMS early in the fourth quarter of 2025 and 24-week data in the first quarter of 2026. We anticipate making a program decision based on these data and the evolving landscape for the development of new therapies for RMS in early 2026.”
“BTK inhibition is a validated mechanism for the treatment of progressive forms of MS, and there is immense scientific interest in its potential to impact inflammation compartmentalized in the CNS and thereby potentially impact disability progression independent of relapse activity. We believe the differentiated, potentially best-in-class profile of orelabrutinib could make a meaningful difference for patients with PPMS and SPMS, which have few treatment options. With our late-stage development capabilities and expertise in MS, we are well positioned to execute on the pivotal, global, Phase 3 development of orelabrutinib, as well as advance two early candidates into clinical development,” said Lisa von Moltke, M.D., Head of Research and Development and Chief Medical Officer of Zenas.
Pipeline Overview
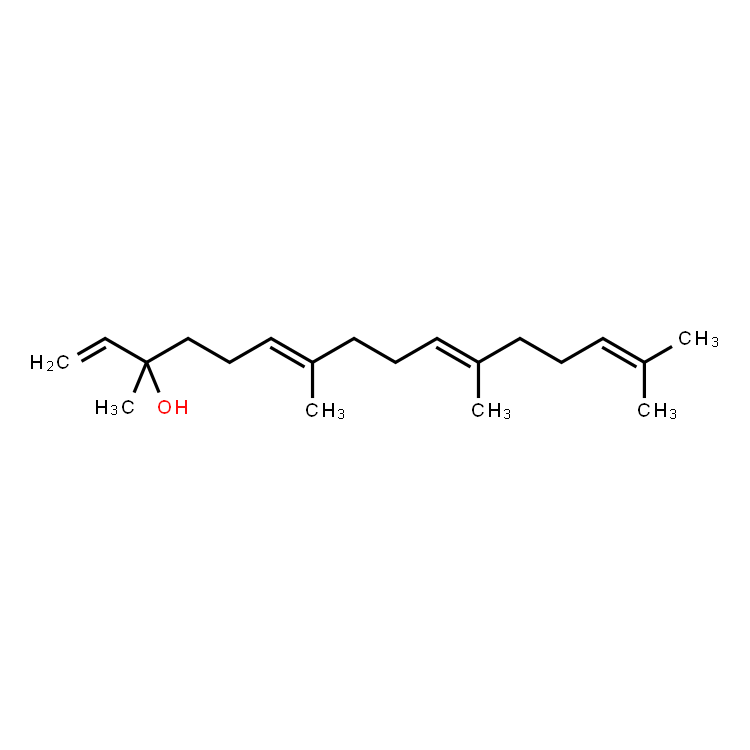
Orelabrutinib, a highly selective CNS-penetrant, oral, small molecule BTK inhibitor
· PPMS Phase 3 trial, a global registration-directed, multicenter, randomized, double-blind, placebo-controlled trial, to evaluate the efficacy and safety of orelabrutinib for patients with PPMS initiated in the third quarter of 2025.
· SPMS Phase 3 trial, a global registration-directed, multicenter, randomized, double-blind, placebo-controlled trial, to evaluate the efficacy and safety of orelabrutinib for patients with SPMS is expected to initiate in the first quarter of 2026.
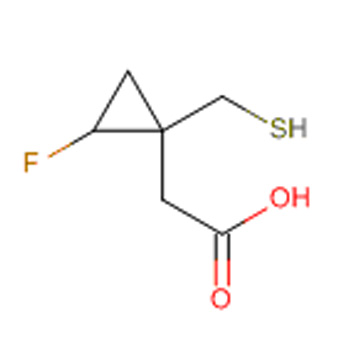
Oral, IL-17AA/AF inhibitor that blocks IL-17 AA homodimer and IL-17AF heterodimer signaling
Currently in Investigational New Drug (IND) enabling studies. Zenas expects to submit an IND and initiate Phase 1 clinical development in 2026.
Oral, brain-penetrant, TYK2 inhibitor
Currently in IND enabling studies. Zenas expects to submit an IND and initiate Phase 1 clinical development in 2026.
Obexelimab, a CD19 and FcγRIIb inhibitor of B cell function
· Immunoglobulin G4-Related Disease (IgG4-RD) Phase 3 INDIGO trial, a global registration-directed, multicenter, randomized, double-blind, placebo-controlled trial, to evaluate the efficacy and safety of obexelimab in patients with IgG4-RD. INDIGO is the largest clinical trial conducted in patients living with IgG4-RD to date. Target enrollment of the INDIGO trial concluded in November 2024, and Zenas expects to report topline results around year-end 2025.
· Relapsing Multiple Sclerosis (RMS) Phase 2 MoonStone trial, a multicenter, randomized, double-blind, placebo-controlled trial, to evaluate the efficacy and safety of obexelimab in patients with RMS. Zenas expects to report results from this trial, including the 12-week primary endpoint results, early in the fourth quarter of 2025.
· Systemic Lupus Erythematosus (SLE) Phase 2 SunStone trial, a multicenter, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of obexelimab in patients with SLE. Zenas expects to complete enrollment of the Phase 2 SunStone trial around year-end 2025 and report topline results in mid-2026.
License Agreement
Under the license agreement, Zenas will pay InnoCare upfront and near-term milestone payments of up to $100 million in cash, including milestone achievements expected in 2026, and up to 7,000,000 shares of Zenas common stock, including shares issuable upon a milestone expected to be achieved in early 2026. The total of the upfront payment, near term milestone and potential development and regulatory milestone payments, along with potential commercial sales achievement milestone payments for all three programs, exceeds $2 billion.
In addition, InnoCare is entitled to receive tiered royalties of up to high teens percentages on annual net sales of the licensed products.
Zenas will have the exclusive right to develop, manufacture and commercialize orelabrutinib in the field of MS globally, and non-oncology fields in all territories outside Greater China and Southeast Asia, while InnoCare retains full global rights in the field of oncology. Zenas will also have the exclusive right to develop, manufacture and commercialize the oral, IL-17AA/AF inhibitor in all territories outside Greater China and Southeast Asia, and the oral, brain-penetrant, TYK2 inhibitor globally.
Private Placement Financing
Zenas has entered into a securities purchase agreement for a private placement financing of shares of its common stock (the "Private Placement"). The Private Placement is expected to result in gross proceeds to Zenas of approximately $120.0 million, before deducting placement agent fees and other Private Placement expenses payable by Zenas.
Pursuant to the terms of the securities purchase agreement, at the closing of the Private Placement, Zenas will issue approximately 6.3 million shares of its common stock to (i) certain institutional and accredited investors at a price of $19.00 per share and (ii) certain directors and officers of the Company at a price of $20.85 per share. The closing of the Private Placement is expected to occur on or about October 9, 2025, subject to the satisfaction of customary closing conditions.
Jefferies and Evercore ISI served as exclusive placement agents for the Private Placement.
The Private Placement included participation from a syndicate of new and existing investors, including mutual funds and healthcare dedicated funds.
Upon closing of the Private Placement, Zenas expects that its cash, cash equivalents and investments will be sufficient to fund its operating expenses and capital expenditure requirements into the fourth quarter of 2026, and assuming receipt of the potential $75 million milestone from Royalty Pharma for the defined success criteria in the Phase 3 INDIGO trial, into the first quarter of 2027.
The sale and issuance of the foregoing shares are being made in a transaction not involving a public offering and have not been registered under the Securities Act of 1933, as amended (the "Securities Act"). The shares being issued in the Private Placement may not be offered or sold in the United States absent registration or pursuant to an exemption from the registration requirements of the Securities Act and applicable state securities laws. Zenas has agreed to file a registration statement with the Securities and Exchange Commission covering the resale of the shares acquired by the investors in the Private Placement.
This press release does not constitute an offer to sell or the solicitation of an offer to buy the securities, nor shall there be any sale of the securities in any state in which such offer or sale would be unlawful prior to the registration or qualification under the securities laws of such state. Any offering of the shares under the resale registration statement will only be by means of a prospectus.
Conference Call Information
InnoCare will host a conference call at 8:30 a.m. Beijing time on October 9, 2025, in Chinese.
Zenas BioPharma will host a conference call and webcast today, October 8, 2025, at 8:00 a.m. ET to discuss the transformational license agreement and to provide an update on the Company’s business and strategy. To access the live webcast of the call, please visit the “Events and Presentations” page in the Investor & Media Relations section of the Zenas BioPharma website. A replay of the webcast will be available following the call.
About Orelabrutinib
Orelabrutinib is a late-stage, potentially best-in-class, highly selective CNS-penetrant, selective, irreversible, oral small molecule Bruton’s Tyrosine Kinase (BTK) inhibitor. In Multiple Sclerosis (MS), InnoCare initiated a Phase 3 trial for Primary Progressive MS (PPMS) in the third quarter of 2025. A Phase 3 trial for Secondary Progressive MS (SPMS) is expected to initiate in the first quarter of 2026. The Phase 3 PPMS and SPMS trials have obtained U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) alignment. Orelabrutinib is approved for B cell malignancies in mainland China and Singapore.
About Obexelimab
Obexelimab is a bifunctional monoclonal antibody designed to bind both CD19 and FcγRIIb, which are broadly present across B cell lineage, to inhibit the activity of cells that are implicated in many autoimmune diseases without depleting them. This unique mechanism of action and self-administered, subcutaneous injection regimen may broadly and effectively address the pathogenic role of the B cell lineage in chronic autoimmune disease.
Obexelimab has been evaluated in six clinical trials in a total of 208 patients who received obexelimab either as an intravenous infusion or as a subcutaneous injection. Obexelimab was well tolerated and demonstrated clinical activity across these clinical trials, providing the Company with an initial clinical proof of concept for obexelimab as a potent B cell inhibitor for the treatment of patients living with certain autoimmune diseases. Zenas is conducting a fully enrolled Phase 3 trial in Immunoglobulin G4-Related Disease and Phase 2 trials for Relapsing Multiple Sclerosis and Systemic Lupus Erythematosus.




































 (All Rights Reserved)
(All Rights Reserved)