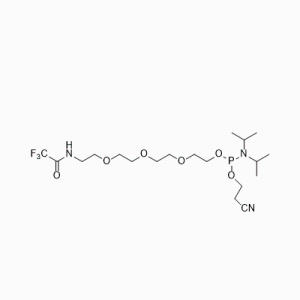

 Sliver Member
Sliver Member
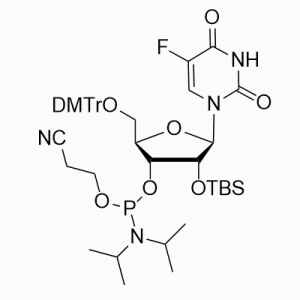
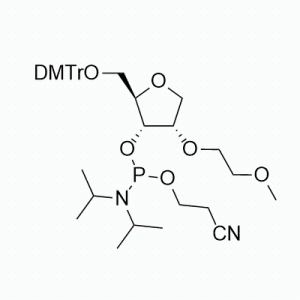
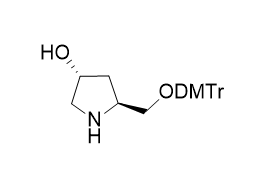
The base part: It contains uracil (U) base, which is an important base in nucleic acids and participates in the transmission and expression of genetic information and other processes in RNA.
The glycoside part: The 2' -hydroxyl group on the glycoside is protected by a tert-butyldimethylsilyl group (OTBS). This protective group can prevent unnecessary reactions of the 2' -hydroxyl group during nucleic acid synthesis, thereby enhancing the selectivity and efficiency of the synthesis. Meanwhile, there is usually an activated phosphoamide group at the 5' -position, which is used for ligation reactions with other nucleotides.
5-fluorine substitution: A fluorine atom (5F) is introduced at the 5-position of uracil. The introduction of the fluorine atom can alter some of the chemical properties and biological activities of the nucleotide, such as possibly affecting its pairing ability with complementary bases and stability against nucleases.
Function and application: Mainly used for the chemical synthesis of nucleic acids (such as DNA or RNA) through the phosphamide method. During the synthesis process, it acts as a monomer unit and gradually connects through a series of chemical reactions to form nucleic acid chains of specific sequences. Due to the protection of its 2' -hydroxyl group, it can ensure that the formation of phosphodiester bonds during the synthesis process has a high degree of regioselectivity, allowing nucleotides to be accurately linked in a predetermined sequence, thereby synthesizing nucleic acid molecules with specific sequences and functions, which can be used to prepare various oligonucleotide drugs, nucleic acid probes, primers, etc.
Synthesis method: Usually, the corresponding 5-fluoro-2 '-deoxyuridine is used as the starting material. Firstly, the 2' -hydroxyl group is protected by tert-butyldimethylsilyl, and then the 5' -hydroxyl group is subjected to phosphoamidation reaction to introduce the phosphoramide group. After multiple steps of reactions, 5F-2' OTBS-U is finally obtained phosphoramidite.
Properties and stability: Under appropriate storage conditions (usually low temperature, dryness, away from light, etc.),5F-2 'OTBS-U phosphoramidite is relatively stable. However, it is relatively sensitive to environmental factors such as water, acids and alkalis. During use and storage, it is necessary to avoid contact with these substances to prevent the shedding of the protective group or the degradation of molecules. At the same time, it should also be avoided from being mixed with strong oxidants, reductants and other substances to prevent chemical reactions that may affect its performance and application.

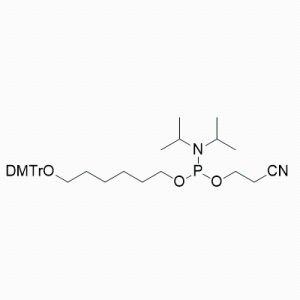
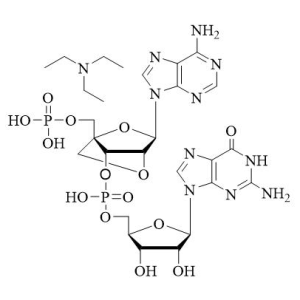
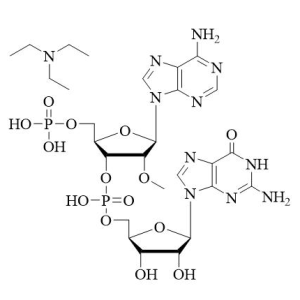
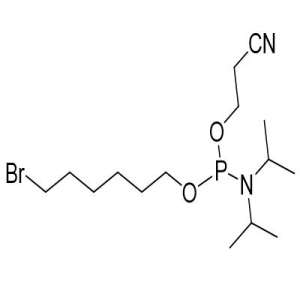
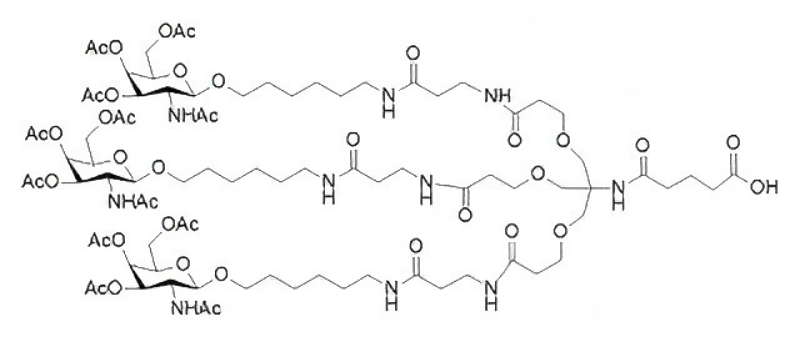
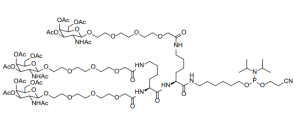
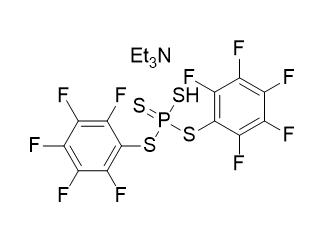
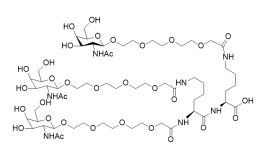














 (All Rights Reserved)
(All Rights Reserved)