
 Standard Member
Standard Member
Hydroxypropyl Betadex Oral pharma grade USP Standard
Product name: Hydroxypropyl Betadex
CAS No.:128446-35-5
DMF No.:030168
Standard :CP / USP /EP
Grade: Oral grade / Pharma grade
Basic information of the oral grade Hydroxypropyl Betadex
Product Name: Hydroxypropyl Betadex oral grade
......................................................................................................................................................
Synonyms: Hydroxypropyl-β-Cyclodextrin; Hydroxypropyl beta Cyclodextrin
......................................................................................................................................................
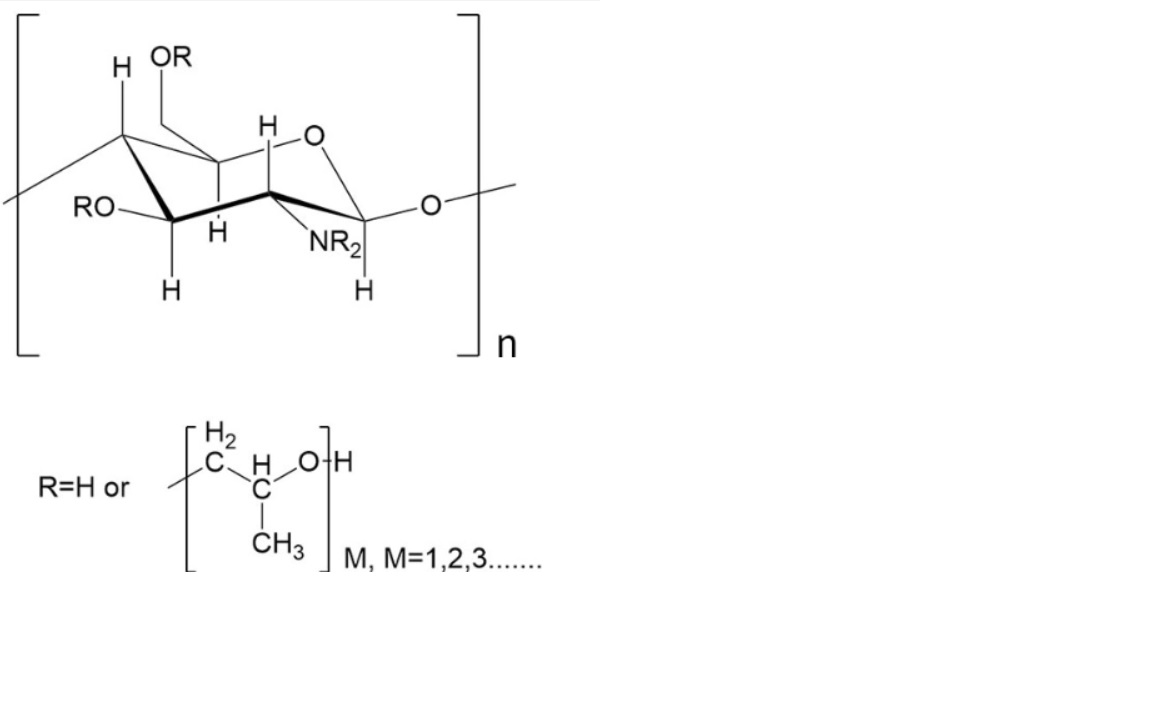
Molecular Formula: C42H70-nO35(C3H8O2)n
......................................................................................................................................................
Molecular Weight: 1134.98+58n
......................................................................................................................................................
Molecular structure:

Product Specification
| Test Standard | USP |
| ITEMS | SPECIFICATION |
| Molar Substitution | 0.40-1.50 |
| Clarity of solution | 50%(w/v) water solution is clear |
| Identification(IR) | same absorption bands as USP Hydroxypropyl Betadex RS |
| Loss on Drying | ≤10.0% |
| Conductivity | 10%(w/v) water solution ≤200µS/cm |
| Propylene oxide | ≤1ppm |
| Propylene glycol | ≤2.5% |
| Sum of Other Impurities | ≤1% |
| Betadex | ≤1.5% |
| Any other single impurity | ≤0.25% |
| TAMC(cfu/g) | ≤1000cfu/g |
| TYMC(cfu/g) | ≤100cfu/g |
Main usage of Hydroxypropyl Betadex Oral pharma grade
-
To increase the solubility of medicine and biological availability
-
To improve the bioavailability of drugs.
-
To adjust or control the releasing of drugs.
-
To decrease the toxicities of drugs.
-
To improve the stabilities of drugs.


























 (All Rights Reserved)
(All Rights Reserved)