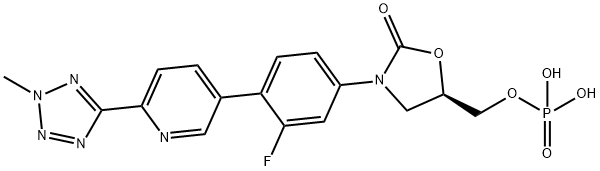
Tedizolidone phosphate (also known as Tedizolidone phosphate, Tedizolidone phosphate, Tedizolidone phosphate) is a second-generation oxazolidone antibiotic developed by Dong-APharmaceutical. In Phase III clinical trials, its clinical efficacy was comparable to that of linezolid, with fewer adverse reactions than linezolid in terms of reduction of gastrointestinal and blood Chemicalbook plates and a lower incidence of resistance. Some experiments have shown that the tolerance of terezolamide is also better than vancomycin. FDA approved dosage forms are available as injections and tablets for clinical switching, and are used once a day for six days, which is more convenient for clinical use than nezolamide twice a day for ten days. Therefore, in view of its good clinical effect, smaller dose and shorter administration cycle, suitable for a wide population and a broad market capacity.


 Standard Member
Standard Member
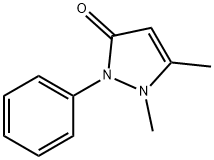

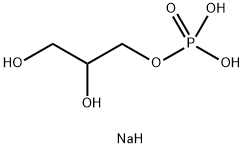





















 (All Rights Reserved)
(All Rights Reserved)