

 Standard Member
Standard Member
Founded in 2007 with a 70 million RMB investment, Cobetter Filtration Validation Center has been accredited by China National Accreditation Service for Conformity Assessment (CNAS). Our Validation Center Team includes 10+ international experts and 80+ lab technicians. We provide over 1,200 technical validation reports annually to customers in the pharmaceutical industry and 1,300 technical validation reports annually to customer in the electronics, chemical, and life sciences (excluding pharmaceutical) industries.
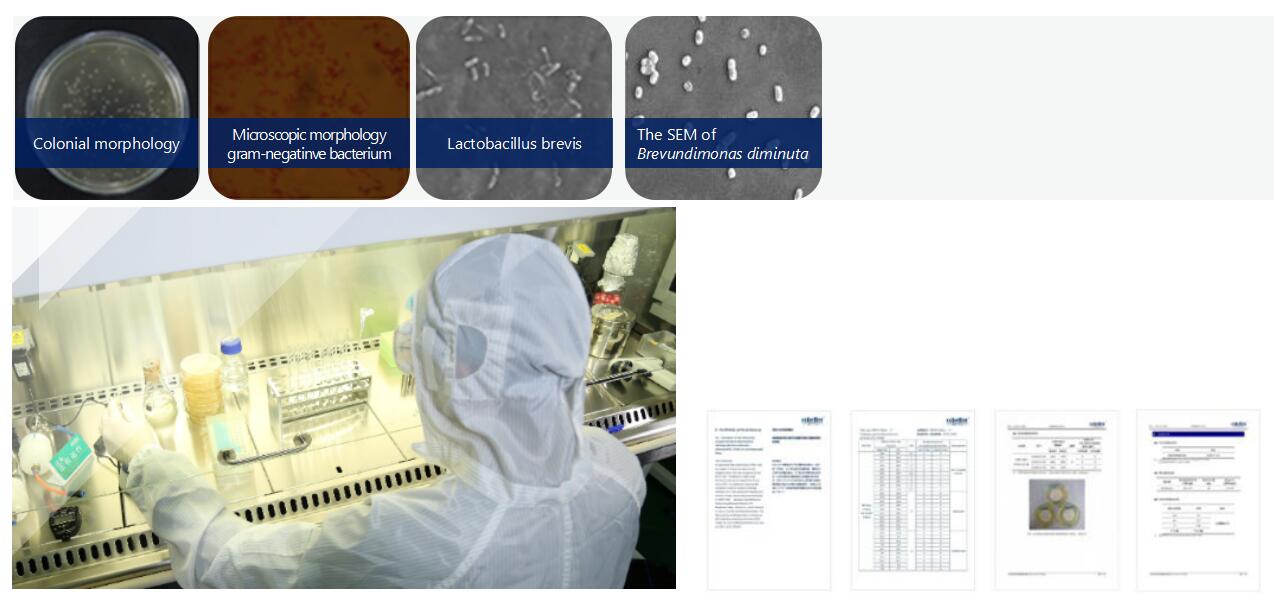
· Bacteria Challenge Test
With our technical experience and deep understanding of the sterilization process, our Validation Center can provide validation services to global customers in the Life Sciences Industries so that they comply with GMP standards regarding Data Integrity.
Validation Services include Bacterial Challenge Test (BCT), Extractable Test, Chemical Compatibility, Integrity Test, and Adsorption Test.
By following both PDA TR26 and ASTM F838 standards, Cobetter Validation Center providers her customers with customized filtration solutions and technical support.
|
Process Validation |
Sterilized Pre-filters |
Sterilized Final Filters |
|
Bacteria Challenge Test |
Required |
Optional |
|
Extractable Test |
Required |
Required |
|
Chemical Compatibility |
Required |
Required |
|
Integrity Test |
Optional |
Optional |
|
Absorption Test |
Evaluate and Perform if Necessary |
Evaluate and Perform if Necessary |






























 (All Rights Reserved)
(All Rights Reserved)