Yes
No



Search Products & Suppliers
Products Directory Suppliers Discovery Post EZSourcingService
Buyer Sourcing Event Contact UsQuick links
My Favorites Recent Visit History
Quantity Requierd: 200Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients
Quantity Requierd: 50Box(es) Expired Date: 2025-10-19
Categories: Finished Dosage
Quantity Requierd: 1000000Vial(s) Expired Date: 2027-07-07
Categories: Finished Dosage > Antibiotics and Antiviral Preparations

Quantity Requierd: 1Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients

Quantity Requierd: 1Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients

Quantity Requierd: 1Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients

Quantity Requierd: 1Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients

Quantity Requierd: 1Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients

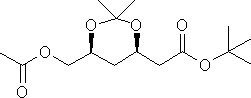
rosuvastatin intermediates C-4
![4-Chloro-6-iodo-7H-pyrrolo[2,3-d]pyrimidine](https://eimg.pharmasources.com/image/product/20201210/d9a52b472b3180d9e6d1d1864813e4ecjpg!280)
4-Chloro-6-iodo-7H-pyrrolo[2,3-d]pyrimidine
![Methyl[E]-2-[3-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-oxopropyl]benzoate](https://eimg.pharmasources.com/image/product/default.jpg!280)
Methyl[E]-2-[3-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-oxopropyl]benzoate

Gelatin Capsules

tri-n-octylphosphine



Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025





 United Kingdom
United Kingdom
 Yemen
Yemen
 Vietnam
Vietnam



