Yes
No



Search Products & Suppliers
Products Directory Suppliers Discovery Post EZSourcingService
Buyer Sourcing Event Contact UsQuick links
My Favorites Recent Visit History
Quantity Requierd: 5Liter(s) Expired Date: 2026-06-30
Categories: Intermediates > Pesticide intermediates

Quantity Requierd: 5000Bottle(s) Expired Date: 2025-12-31
Categories: Bio Products > Protein/Antigen/Polypeptide > Protein Synthesis
Quantity Requierd: 400Kilogram(s) Expired Date: 2025-09-20
Categories: Active Pharmaceutical Ingredients
Quantity Requierd: 300Kilogram(s) Expired Date: 2025-09-20
Categories: Active Pharmaceutical Ingredients
Quantity Requierd: 200Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients
Quantity Requierd: 50Box(es) Expired Date: 2025-10-19
Categories: Finished Dosage
Quantity Requierd: 1000000Vial(s) Expired Date: 2027-07-07
Categories: Finished Dosage > Antibiotics and Antiviral Preparations

Quantity Requierd: 1Kilogram(s) Expired Date: 2025-09-30
Categories: Active Pharmaceutical Ingredients


Metronidazole
Mesitylene(1,3,5-Trimethylbenzene)
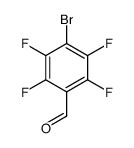
4-Bromo-2,3,5,6-tetrafluorobenzaldehyde

Chitosan for the clarification of floc

Hydroxyethyl Cellulose(HEC)



Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025





 Turkey
Turkey
 United Kingdom
United Kingdom
 Yemen
Yemen
 Vietnam
Vietnam



