 Romania
Romania

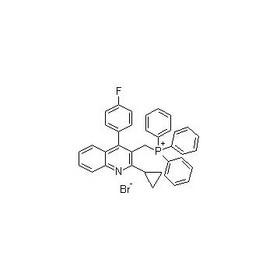
3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinolone

Midazolam Maleate

Penehyclidine

Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


