August 15, 2025
Tag:
1 Preface
Sodium silicate, commonly known as water glass, is a water-soluble silicate. Its aqueous solution is commonly called water glass and is a kind of mineral adhesive. Industrial sodium silicate is divided into solid industrial sodium silicate and liquid industrial sodium silicate. Solid sodium silicate is mainly used to produce liquid sodium silicate, while liquid sodium silicate is mainly used as a chemical raw material, filler, binder, additive, preservative, etc. Therefore, its quality grade plays a decisive role in application. How to efficiently and quickly detect its content index is a matter of great concern to manufacturers. This scheme is based on the detection method of industrial sodium silicate in GB/T 4209-2008. It uses the JH-T9 fully automatic potentiometric titrator for detection. With multi-module liquid addition, it avoids the cumbersome step of changing the titrant, takes less time, and avoids the subjective error caused by manual judgment of the endpoint. It is the preferred choice for detecting its content.
2. Instruments and equipment
2.1 Instruments
JH-T9 fully automatic potentiometric titrator, composite PH electrode, constant temperature reaction vessel, constant temperature drying oven.
2.2 Reagents
NaOH standard titrant (0.1mol/L), hydrochloric acid standard titrant (0.1mol/L), hydrochloric acid solution (1mol/L).
3 Experimental Methods
3.1 Experimental Procedures
1. Sample pretreatment work:
1) Place the received samples in a constant temperature drying oven at 110℃ for drying for two hours. Take out the dried samples and put them in a crusher for grinding. Then, place the ground samples in a 110℃ drying oven until the mass weight is constant.
2) Weigh 1g of the dried sample, with an exact value of 0.0001g, and place it in a hydrothermal reactor. Add 5mL of water and place it under electric heating
Keep at 180℃ in a constant temperature drying oven for 2 hours. After the experiment is completed, cool down to 40℃, dissolve the sample in water at 80℃, transfer it to a 250mL volumetric flask, make up to the mark, and wait for the test.
2. Titration process:
1) Electrode calibration: Calibrate the PH electrode with buffer solutions of PH=4.01, 6.86, and 9.18 for subsequent testing.
2) Sodium oxide content test:
Transfer 50mL of the dissolved sample from a 250mL volumetric flask with a 50mL pipette and place it in a titration cup. Put the rotor in the titration cup and place it on the titration stand. Start Method one and use the first module to titrate with the calibrated hydrochloric acid titrant until PH=4.4, reaching the endpoint and completing the titration.
3) Determination of silica content:
After the above titration experiment is completed, remove the titration cup and add 3g±0.1g of sodium fluoride (ensuring excess) that has been weighed in advance, to
Dissolve it, then accurately transfer 10 ml of the calibrated 1mol/L hydrochloric acid solution (ensuring excess) with a 10mL pipette. After thorough mixing, start Method two and use the second module to titrate with the calibrated sodium hydroxide titrant until PH=6.2 is reached, and the titration is completed. At the same time, conduct a blank experiment. In the titration cup, add 3g of sodium fluoride to dissolve it, then add a known excess of salt acid, and titrate with sodium hydroxide until the PH=6.2.
3.2 Parameter Settings
Method One
| Titration mode | endpoint titration | Equilibrium potential before titration |
6mV |
| The stirring speed |
7 |
the final volume |
20mL |
| Pre-add volume |
15mL |
pre-stir time |
5s |
| The fast drop volume |
0.2mL |
the slow drop volume |
0.02mL |
| The fast drop potential equilibrium time |
4s |
the fast drop equilibrium potential |
1mV |
| The slow drop potential equilibrium time |
4s |
the slow drop equilibrium potential |
1mV |
| Titration endpoint |
6.2 |
Pre-control value |
6(Delay for 10 seconds ) |
Method Two
| Titration mode | endpoint titration | Equilibrium potential before titration |
6mV |
| The stirring speed |
7 |
the final volume |
20mL |
| Pre-add volume |
15mL |
pre-stir time |
5s |
| The fast drop volume |
0.2mL |
the slow drop volume |
0.02mL |
| The fast drop potential equilibrium time |
4s |
the fast drop equilibrium potential |
1mV |
| The slow drop potential equilibrium time |
4s |
the slow drop equilibrium potential |
1mV |
| Titration endpoint |
6.2 |
Pre-control value |
6(Delay for 10 seconds ) |
4. Results and Discussion
4.1 Experimental Results
1) Determination of sodium oxide content
| Sample name | Sampling volume | Titration volume |
Content( % ) |
Average content |
RSD(%) |
|
Industrial silicic acid sodium 1# |
1.00287 |
14.417 |
23.033 |
23.063 |
0.1241 |
|
14.438 |
23.066 |
||||
|
14.453 |
23.090 |
||||
|
Industrial silicic acid sodium 2# |
1.00399 |
14.402 |
22.983 |
22.903 |
0.5725 |
|
14.257 |
22.752 |
||||
|
14.397 |
22.975 |
2) Determination of silica content:
| Sample name | Sampling volume | Titration volume |
Content( % ) |
Average content |
RSD(%) |
|
Industrial silicic acid sodium 1# |
1.0028 7 |
15.971 |
78.978 |
79.089 |
0.132 |
|
15.677 |
79.185 |
||||
|
15.790 |
79.105 |
||||
|
Industrial silicic acid sodium 2# |
1.0039 9 |
16.281 |
78.700 |
78.369 |
0.374 |
|
16.860 |
78.264 |
||||
|
17.034 |
78.142 |
Calculation formula
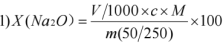
In the formula:
V is the volume of the standard titrant of hydrochloric acid consumed, with the unit of mL;
c is the concentration of the calibrated hydrochloric acid titrant, with the unit of mol/L;
m is the mass of the dissolved sample, with the unit of g.
M is the molar mass value of sodium oxide (1/2Na2O), with the unit g/mol (M=30.99).
Jiahang
c1 is the concentration of the hydrochloric acid standard solution, with a value of 1.2014, and the unit is mol/L. c2 is the concentration of the sodium hydroxide standard titrant, with the unit of mol/L;
V1 is the volume of the standard hydrochloric acid solution consumed in the titration, with a value of 10 and the unit of mL.
V2 represents the volume of sodium hydroxide standard titrant consumed during titration, with the unit of mL.
V3 is the standard of hydrochloric acid consumed in the blank, the volume of the liquid, with a value of 0.3, and the unit is mL. V4 represents the volume of sodium hydroxide standard titrant consumed in the blank, with a value of 4.320 and the unit being mL.
M is the molar mass value of silicon dioxide (1/4SiO2), with the unit g/mol (M=15.02).
m is the mass of the dissolved sample, with the unit of g.
4.2 Atlas
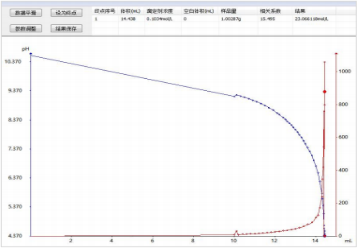
1) Titration spectrum for sodium oxide content determination:
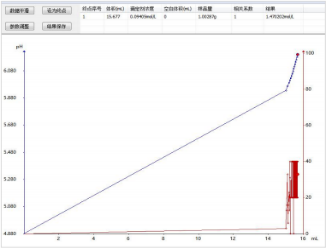
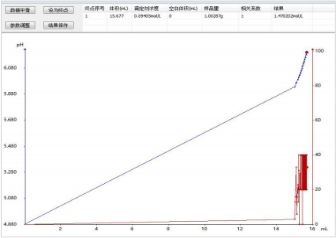
3) Titration spectrum for silica content determination:
4.3 Conclusion
The content of industrial sodium silicate determined by the JH-T9 fully automatic potentiometric titrator has good repeatability, and all the determination results are within its standard range. The JH-T9 fully automatic potentiometric titrator fully meets the determination requirements of this sample.
Notes
The endpoint is to titrate to a definite PH value, so the electrode must be calibrated with a buffer solution before the experiment.
2. The endpoints 4.4 and 6.2 of this experiment were determined by adding 10 drops of methyl red indicator before the titration began and observing the color change of the indicator. Due to subjectivity, there may be slight deviations, which can be adjusted appropriately.
References
[1] GB/T 4209 Industrial sodium silicate [M].


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


