PharmaSources/1℃March 18, 2020
Tag: immunotherapies , HCC , sorafenib
As a high burden in the world, hepatocellular carcinoma (HCC) mainly occurs in HBV, HCV and alcoholic liver cirrhosis patients. The new cases reach nearly 750,000 ever year in the world[1]. HCC belongs to a rare disease in countries or regions such as Europe and the U.S., however, in Asia, especially China, HCC has a high incidence with a 5-year survival rate of only 11%[2].
Opdivo kicked off the era of HCC immunotherapies in the world, and AiRuiKa’s taking the lead in receiving the approval for HCC indication in China was an important milestone!
Major First-line/Second-line Therapies for Advanced HCC[2].
![Major First-line/Second-line Therapies for Advanced HCC[2].](https://cimg.cphi.cn/img_Cphi_cn/news/2020_03/Mimg_2003181024718892.png)
The era of multikinase inhibitors: sorafenib and lenvatinib become the first-line standard therapies of HCC
Sorafenib was the first drug approved as the first-line therapy in HCC in 2007: it could reduce the death risk of patients by 31%.
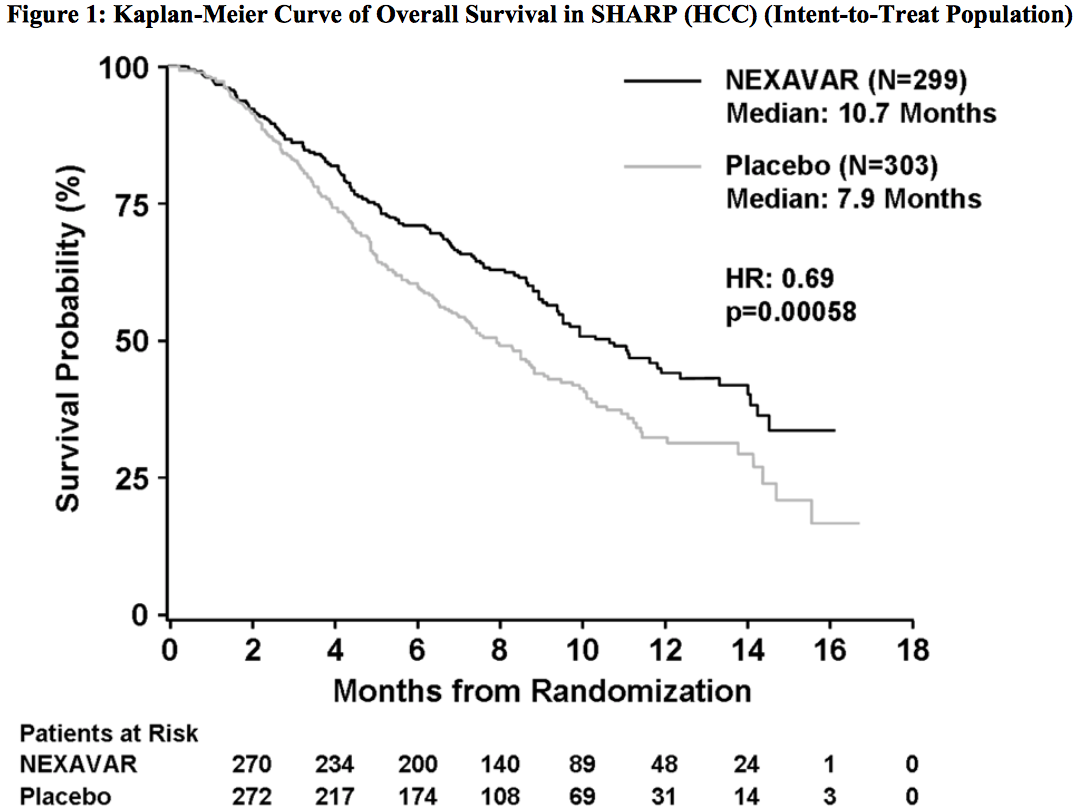
Source: Sorafenib prescribing-information
Lenvatinib showed non-inferiority vs sorafenib in the overall survival (OS): it was approved as a first-line therapy in HCC in 2018.
Source: Lenvatinib prescribing-information
Donafenib showed superiority vs sorafenib: it is the first small molecule drug superior to sorafenib in recent 12 years.
Donafenib was announced important clinical progress on Jan. 1, 2020 that the CTR20160184 registered phase III clinical trial of first-line treatment of advanced HCC (trial code ZGDH3, CTR20160184) was successful; in inoperable or metastatic advanced HCC patients who had not received systemic treatment, the median overall survival (mOS) of the donafenib group was significantly better than the controlled sorafenib group and reached a statistically significant difference and an extension of clinical significance. It is the first small molecule drug with the OS superior to sorafenib in recent 12 years! If you are looking for healthcare supply companies offering this kind of drug, then Pharmasources would be your best choice.
The era of tumor immunotherapies: anti-PD-(L)1 monoclonal antibodies bring breakthroughs to HCC treatment
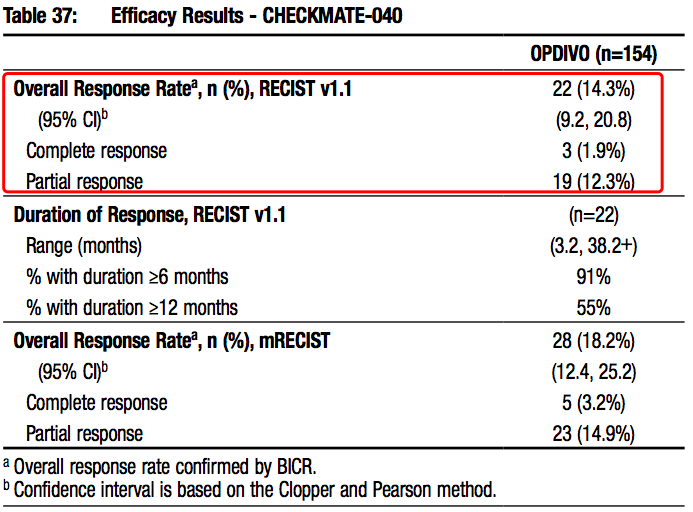
Opdivo was approved as a second-line therapy of HCC upon CheckMate-040 in 2017. It was the first anti-PD-(L)1 antibody approved for this indication, marking the start of the era of tumor immunotherapies of HCC!
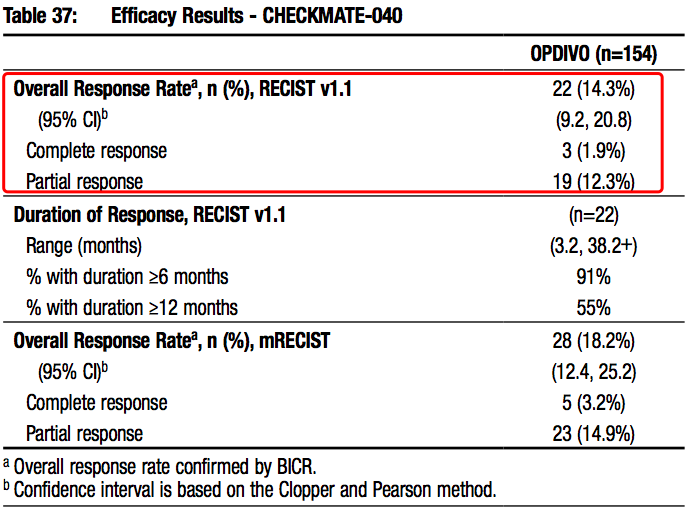
Source: Opdivo prescribing-information
Keytruda was approved as a second-line therapy in HCC upon KEYNOTE-224 in 2018 to become the world’s 2nd anti-PD-(L)1 antibody approved for this indication.
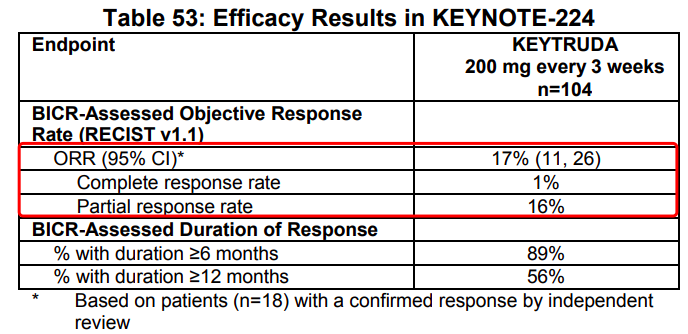
Source: Keytruda prescribing-information
Tumor immunotherapies: Tecentriq + Avastin to become a breakthrough in the HCC first-line therapy in recent 12 years
Oct. 21, 2019 was a memorable day!
Roche announced milestone research progress: according to the data of IMbrave150, compared to sorafenib, Tecentriq + Avastin could bring significant OS and progression-free survival benefits to unresectable HCC patients and reduce the death risk of HCC patients by 42%, showing superiority!!!
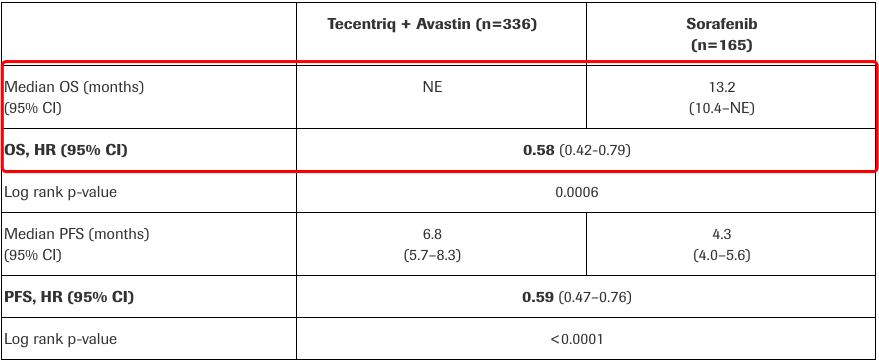
Source: Roche
Hengrui Medicine’s AiRuiKa to kick off the era of tumor immunotherapies of HCC in China
CTR20160871 (NCT02989922);
AiRuiKa could reach an overall response rate (ORR) of 14.7% and a 6-month OS rate of 74.4% in HCC patients.
Refer to DOI: https://doi.org/10.1016/S1470-2045(20)30011-5 for the detailed data.
Summary of HCC Therapeutic Regimens:
| Regimen | Category |
First-line therapy | Sorafenib, lenvatinib, anti-PD-(L)1 monoclonal antibody* | Multikinase inhibitor |
Second-line therapy | Anti-PD-(L)1 monoclonal antibody, cabozantinib, ramucirumab | Monoclonal antibody, multikinase inhibitor |
*Namely, Roche’s regimen of Tecentriq + Avastin, which is currently at the marketing review stage.
Future focuses on the tumor immunotherapies as first-line therapies of HCC
Registration No. | Trial group vs. positive control | Description |
CTR20181039 NCT03605706 | Camrelizumab + FOLFOX4 vs. FOLFOX4+ sorafenib | PD-(L)1 |
CTR20182528 NCT03764293 | Camrelizumab + apatinib vs. sorafenib | PD-(L)1 + anti-VEGF |
CTR20190249 NCT03713593 | Pembrolizumab + lenvatinib vs. lenvatinib | PD-(L)1 + multikinase inhibitor |
CTR20182530 NCT03794440 | Sintilimab + IBI305 vs. sorafenib | PD-(L)1 + anti-VEGF |
CTR20180607 NCT03298451 | Durvalumab + Tremelimumab vs. sorafenib | PD-(L)1 + CTLA-4 |
CTR20170882 NCT03412773 | Tislelizumab vs. sorafenib | PD-(L)1 |
NCT04039607 | Nivolumab + ipilimumab vs sorafenib/lenvatinib | PD-(L)1 + CTLA-4 |
CTR20192524 | CS1003 + lenvatinib vs. lenvatinib | PD-(L)1 + multikinase inhibitor |
Chinese liver cancer patients account for more than half in the world: the first-line therapy of Tecentriq + Avastin following the footsteps of camrelizumab.
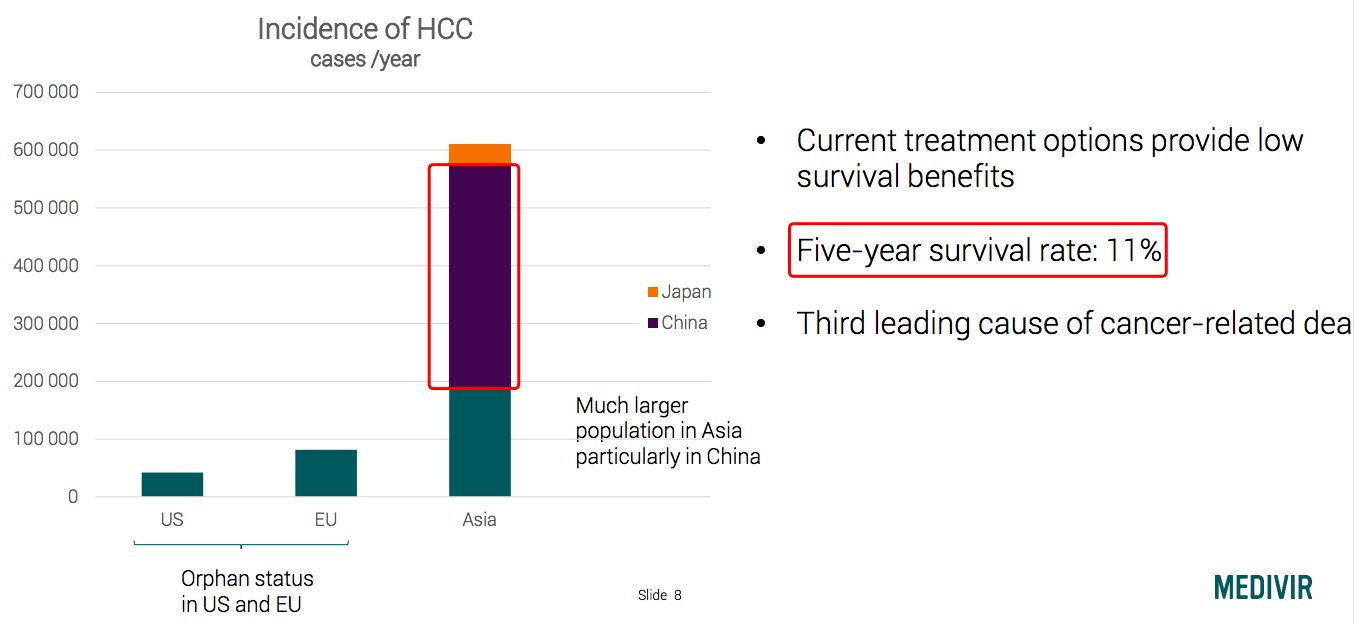
Source: Medivir
![Source: Hepatocellular carcinoma[1]](https://cimg.cphi.cn/img_Cphi_cn/news/2020_03/Mimg_2003181025432104.gif)
Source: Hepatocellular carcinoma[1]
The global burden of HCC. The incidence of hepatocellular carcinoma (HCC) is shown. The main risk factors for HCC development are hepatitis C virus (HCV) infection (for example, Egypt), hepatitis B virus (HBV) infection (China), alcohol intake, non-alcoholic steatohepatitis (NASH; Unites States) and aflatoxin B1 ingestion (Sudan). Mongolia has the highest incidence of HCC globally. HBsAg, hepatitis B surface antigen. Data from Globocan 2012 (REFS 1,3–9). Adapted from REF. 10, Nature Publishing Group.
The market size of liver cancer drugs will double in the next 5 years, and the Tecentriq regimen will become the drug with the fastest growth.
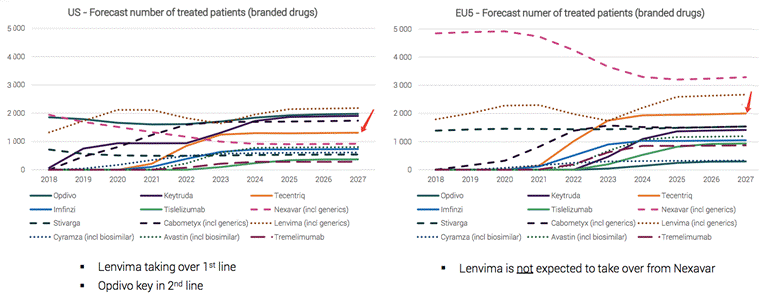
Source: Medivir
As mentioned above, China has a high liver cancer burden, and its huge clinical demands are not satisfied. China will embrace the two HCC first-line therapeutic regimens of Tecentriq and donafenib in the next 1-2 years, which will mark significant progress. The tumor immunotherapies represented by Chinese anti-PD-(L)1 antibodies are expected to achieve breakthroughs in the future, to further improve the choice of first-line therapies in HCC and promote the accessibility of innovative regimens!
[1] Llovet JM et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018
[2] https://www.medivir.com/media/1813/medivir-rnd-day-2-march-2020-f.pdf
-----------------------------------------------------------------------
Editor's Note:
En-CPhI.CN is a vertical B2B online trade platform serving the pharmaceutical industry,
for any copyright disputes involved in the articles,
please email: Julia.Zhang@imsinoexpo.com to motify or remove the content.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


