July 28, 2025
Tag:
1 Preface
Disinfectants are the most commonly used drugs in aquaculture, used to eliminate or kill pathogenic microorganisms and other harmful microorganisms in the external environment. According to their chemical properties, they can be classified into aldehyde disinfectants, alkali disinfectants, iodine disinfectants, bromine disinfectants, chlorine disinfectants, etc. The compound iodine in iodine-based disinfectants is compounded from iodine, phosphoric acid and surfactants, etc. It is a disinfectant with good stability and disinfection effect as tested by the Ministry of Agriculture. According to the "Veterinary Drug Quality Standard" (2017 Edition), it should contain 1.8% to 2% active iodine and 16% to 18% phosphoric acid. In order to establish the internal control quality standard for the complex iodine solution, it is necessary to determine the experimental content of phosphoric acid.
In this paper, two potentiometric titrators, JH-T6 and JH-T7, were respectively selected and tested with different pH electrodes.
2. Instruments and equipment
2.1 Instruments
JH-T6, JH-T7 fully automatic potentiometric titrators 10mL and 15mL burettes; Russell pH composite electrode Hamilton pH composite electrode
2.2 Reagents
NaOH solution (0.1mol/L), ultrapure water, and complex iodine solution
3 Experimental Methods
3.1 Experimental Procedures
Accurately weigh approximately 0.5g of the sample into the titration cup, add 50mL of ultrapure water, and use the stirring function of the instrument to dissolve it completely. Titrate with NaOH solution until the endpoint. At the same time, conduct blank experiments.
3.2 Parameter Settings
(1) JH-T6
|
Titration mode : |
Macro titration |
display unit : |
pH |
|
Endpoint count : |
1 |
Minimum addition volume : |
0.02mL |
|
Final volume : |
15mL |
Initial volume addition : |
0.5mL |
|
Finish jump : |
100 |
|
|
( 2 )JH-T7
| Titration mode: | Dynamic titration | stirring speed |
6 |
| Electrode equilibrium time |
20s |
pre-stirring time : |
8s |
| Electrode equilibrium potential: |
0.5mv |
titration speed: | fast |
| Minimum addition volume: |
0.02mL |
Pre-titration addition volume |
5mL |
| Final volume: |
30mL |
Pre-titration stirring time: |
6s |
| Equilibrium potential before titration: |
50mV |
liquid filling rate: |
6 |
| Potential spike: |
100 |
Pre-controlled pH value: |
2.5 |
4. Results and Discussion
4.1 Results
Concentration of NaOH solution: 0.1053mol/L, blank volume: 0.05mL.
| Instrument Model | Electrode | Sample Volume (g) | Titration volume (mL) | Content (%) | Average Value (%) |
|
T6 |
Russell pH composite electrode Hamilton pH composite electrode Hamilton pH composite electrode |
0.50899 |
8.565 |
17.3 |
17.25 |
|
0.49558 |
8.314 |
17.2 |
|||
|
0.50194 |
8.502 |
17.4 |
17.35 |
||
|
0.54339 |
9.157 |
17.3 |
|||
|
T7 |
0.51364 |
8.707 |
17.4 |
17.42 |
|
|
0.51873 |
8.818 |
17.4 |
|||
|
0.51112 |
8.667 |
17.4 |
|||
|
0.51639 |
8.757 |
17.4 |
|||
|
0.52036 |
8.826 |
17.4 |
|||
|
0.51776 |
8.815 |
17.5 |
Calculation formula
X = (V1 - V0) × c × 9.8 m
In the formula:
X - Content of phosphoric acid, %;
V1 - The consumption of NaOH solution when titrating the sample, in mL;
V0 - The consumption of NaOH solution when titrating the blank, mL;
9.8 - Every 1mL of NaOH solution (0.1mol/L) is equivalent to 9.800mg of phosphoric acid.
m - Mass of the sample, g.
4.2 Atlas
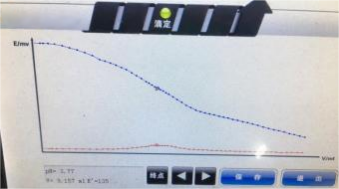
T6 fully automatic potentiometric titrator
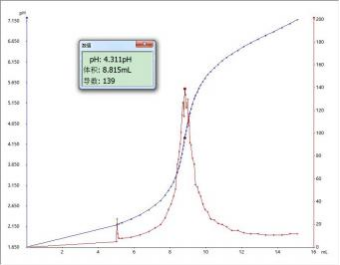
(2) T8 Fully automatic potentiometric titrator
4.3 Conclusion
In this experiment, samples were tested using T6 and T7 fully automatic potentiometric titrators respectively. After being combined with domestic or imported pH composite electrodes, the results showed that they were all around 17.3% -17.4%, which was within the range of phosphoric acid content marked on the complex iodine solution (16%-18%). However, since the T860 interface does not have a zooming function, the potential trend and sudden jump changes in the figure are not obvious. It is recommended to choose the T960 model for the experiment.
Precautions
1. Precise weighing: Place the titration cup directly on the balance with a scale of one ten-thousandth, zero it, and then directly add the sample for weighing. Since weighing 1.0g would lead to an excessive consumption of the solution, in this experiment, we uniformly weighed around 0.5g.
2. Stirring: The T6 instrument is equipped with magnetic stirring, while the T7 instrument is equipped with stirring paddles.
3. NaOH solution needs to be calibrated before use, and the initial potential mV value is generally around 170.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


