June 17, 2025
Tag:
1. Preface
Sodium chloride is indispensable for the human body. It is of great significance for maintaining normal osmotic pressure of cells, regulating the body's acid-base balance, and preserving the physiological activity of proteins.
This method, in accordance with the provisions of the 3107 Sodium Chloride Determination Method in the 2020 Edition of the Chinese Pharmacopoeia, removes the protein from the sample, completely reacts the chloride ions in the solution with an excess of silver nitrate, and titrates the excess silver nitrate with ammonium thiocyanate titrant. After adopting the JH-T7 fully automatic potentiometric titration method of Shanghai Jiahang, it is more accurate, efficient and safe than manual titration, and the data repeatability is good, meeting the detection requirements in the pharmacopoeia.
2 Instruments and reagents
2.1 Instruments
JH-T7 fully automatic potentiometric titrator, silver composite electrode, 10mL burette
2.2 Reagents
Ammonium thiocyanate titrant (0.05mol/L), silver nitrate solution (0.1mol/L), nitric acid solution (8.0mol/L).
3 Experimental Methods
3.1 Experimental Procedures
Accurately measure 1.0mL of the test sample, precisely add 5mL of 0.1mol/L silver nitrate solution, mix well, add 10mL of 8.0mol/L nitric acid solution, heat and digest until clear, cool, and titrate with ammonium thiocyanate titrant (0.05mol/L) until the potential jump endpoint. Correct the titration result with a blank. The titration result was corrected with a blank.
3.2 Instrument Parameters
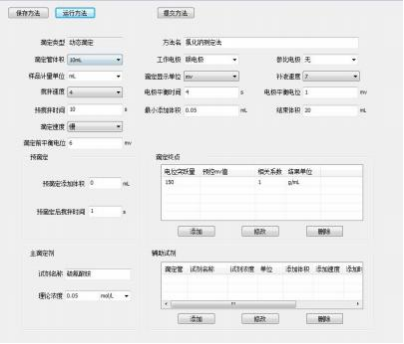
4 Results and Discussion
4.1 Experimental Data
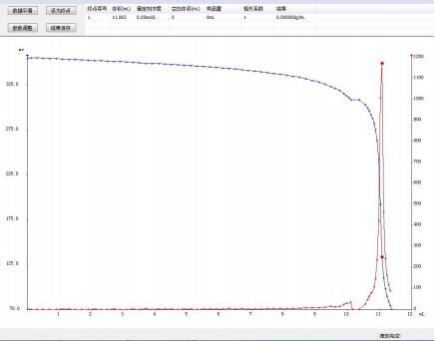
4.1.1 Blank Calibration
| Sample name | titration solution | concentrated sample volume | average titration volume | Average value |
|
Silver nitrate |
0.05 |
5 |
11.083 |
11.098 |
|
11.113 |
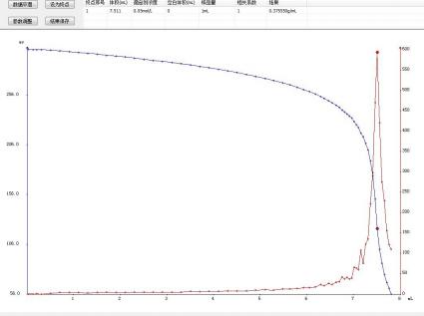
4.1.2 Determination of Aluminum solution
| Sample Name | titration solution | concentrated sample volume | titration volume | Sodium chloride content | Average value |
|
Sodium chloride sample dissolved
liquid |
0.05 |
1 |
7.596 |
10.2346 |
10.2317 |
|
7.511 |
10.4830 |
||||
|
7.684 |
9.9774 |
4.2 Calculation Formula
Sodium chloride content (g/L) = (V0- V X) × c × 58.45, where V0 is the volume of ammonium thiocyanate titrant consumed in the blank test, mL;
VX- The volume of ammonium thiocyanate titrant consumed by the sample, in mL;
C- Concentration of ammonium thiocyanate titrant, mol/L;
V2- The volume of the sample, mL;
58.45 is the molecular weight of sodium chloride.
4.3 Titration Spectrum
4.3.1 Blank
4.3.2 Sodium chloride solution sample
4.4 Conclusion
The determination of sodium chloride content in protein-containing samples by JH-T7 fully automatic potentiometric titrator has accurate results, good data repeatability, is faster and more convenient, and meets the detection requirements.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


