Krebs QinMay 06, 2024
Tag: odronextamab , FDA , market entry
Accelerated approval is a privilege that every applicant desires. Not only can you expedite market entry, but you can also temporarily bypass the time-consuming process of obtaining data for conventional endpoints by leveraging surrogate endpoints to achieve this goal. Once it goes on the market, it means that R&D investment can be recovered, regardless of whether the drug is really effective and safe. As for when to submit post-marketing confirmatory research data, you only need to make a time commitment to FDA, and then you can take your time to make money by listing your products while collecting data for the FDA to see. What should be done in case of exceeding the time limit? You can apply for an extension and continue working. After all, this drug may already have a large patient population, especially for those indications with no other options, and the FDA is likely to be in a dilemma because of its fear of harming the patient. For example, the cancer drug Folotyn (Pralatrexate) received accelerated approval from FDA 15 years ago for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL), but 15 years later, FDA still has no conclusive evidence for the effectiveness of this expensive drug. What's more, Folotyn has changed hands several times in the post-marketing process, but the data is still pending. And its previous and current owners have pushed back deadlines for post-marketing research at least 10 times. According to GoodRx, the annual cost of Folotyn is approximately USD 900,000, and the drug has undergone four price increases since January 2022. And such examples are everywhere in the United States.
But that is a bygone era of yore. The FDA has woken up.
On December 29, 2022, President Biden signed the Consolidated Appropriations Act, 2023, which includes the Food and Drug Omnibus Reform Act (FDORA). In the face of certain developers employing stalling tactics in the post-marketing confirmatory research, two provisions of the newly enacted FDORA law warrant attention. First, FDA is allowed to require pharmaceutical companies to conduct confirmatory research at the same time as obtaining accelerated approval. Otherwise, FDA will have the power to reject the application even if the data is perfect. Regeneron became the first "tester" of this regulation. On March 25, 2024, Regeneron's two applications for CD20xCD3 bispecific antibody odronextamab for relapsed/refractory follicular lymphoma (FL) and relapsed/refractory diffuse large B-cell lymphoma (DLBCL) were rejected by FDA. The issues emphasized in the FDA's complete response letter regarding the confirmatory research of odronextamab do not pertain to efficacy and safety, clinical design, or CMC. Rather, the concern lies in the timeline of the confirmatory tests for odrone xtamab, which did not demonstrate that the confirmatory research was "underway" concurrently with its sBLA application. The CRL received by Regeneron may be the first time in the history of FDA to be issued due to "post-marketing research timetable issues". This phenomenon clearly sent a signal that FDA was tightening its accelerated approval regulatory policy, and also verified the previous commitment of Richard Pazdur, director of FDA's Oncology Center of Excellence, to take a tougher stance on the confirmatory test requirements for new anti-cancer drugs before accelerated approval.
The second major power granted to FDA by FDORA is to simplify the withdrawal procedure, allowing it to withdraw approval directly without holding a public hearing. The first to try this approach was Oncopeptides' multiple myeloma drug Pepaxto (Melphalan flufenamide). Pepaxto received accelerated approval from FDA on February 26, 2021. After confirming the new test, FDA voted (14: 2) against the drug's benefit-risk profile at an advisory committee meeting in September 2022. In July 2023, FDA proposed that Oncopeptides withdraw its drug from the market of the United States. In August, Oncopeptides appealed the FDA's fast withdrawal proposal. In February 2024, FDA officially withdrew its accelerated approval decision for Pepaxto. FDA took7 months from the proposed withdrawal to the formal withdrawal, setting a record for the fastest withdrawal of accelerated approval. The uniqueness of Pepaxto lies in being the inaugural instance where FDA exercised newly granted powers through FDORA to rescind regulatory approval. This manifestation was notably characterized by a prompt withdrawal made swiftly without convening a formal hearing, demonstrating a trend of accelerated approvals swiftly followed by abrupt rescissions (Figure 1). With the endorsement of FDORA, the phenomenon of expedited approval projects transitioning swiftly from inception to cessation could potentially become routine.
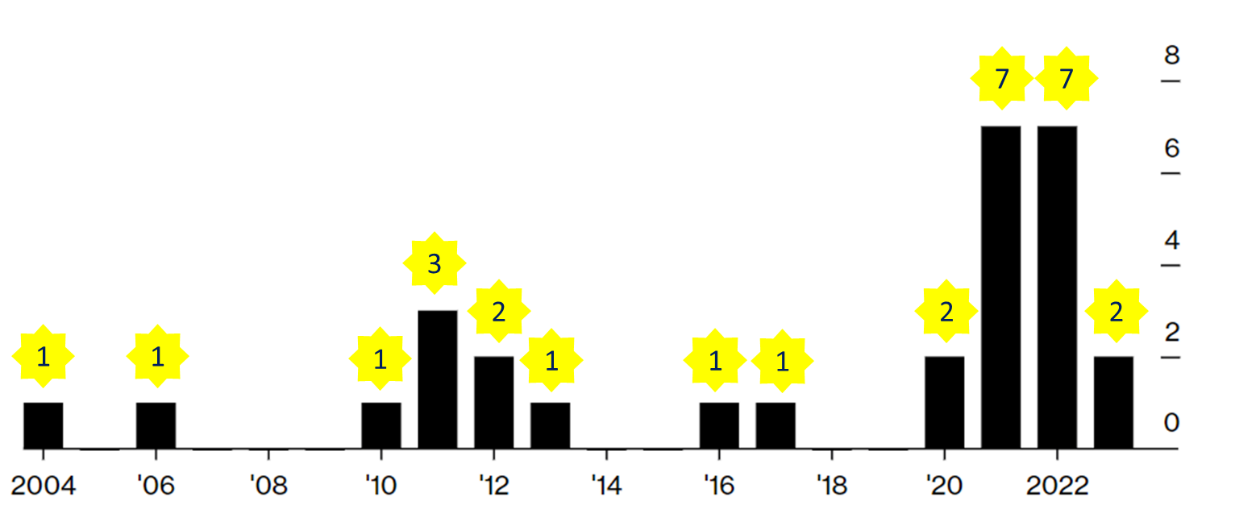
Figure 1 Distribution of Years of Market Withdrawal for Accelerated Approval Drugs (Data Source: Bloomberg). Note: The count includes drugs that have completely exited the market as well as those that have partially withdrawn for alternative purposes. For drugs with multiple approvals, withdrawals occurring in different years are tallied annually.
FDA's two formidable regulatory actions this year regarding accelerated approvals signify a departure from its previous hesitance on the matter, showcasing a newfound agility and assertiveness.
So for developers who want to continue to take the accelerated approval path, in the face of the awakened FDA, how should they be cautious in words and deeds, and take the same accelerated approval path without ending up in despair?
History serves as a valuable guide!
One of the main reasons for the deprivation of accelerated approval drugs is the incompleteness of confirmatory tests. Accelerated approval is a conditional regulatory decision, and the most important thing is to demonstrate the safety and/or efficacy of an approved product through the post-marketing confirmatory research.
According to the research in 2021, drugs that already have interim analysis data from confirmatory tests at the time of accelerated approval are more likely to ultimately receive full approval. Biopharmaceutical developers now need to contemplate how to optimize their planning and resource allocation to successfully complete confirmatory tests. Furthermore, FDORA mandates that confirmation of ongoing validation must coincide with obtaining accelerated approval. While there may be some leeway in the interpretation of this "ongoing" concept, to judge without discernment is to err in both severity and leniency, with odronextamab from Regeneron serving as the latest example.
A major critique within the industry regarding accelerated approvals lies in the criticism of surrogate endpoints rather than directly targeting the essential outcomes, namely those clinically meaningful and patient-centric indices at the core of understanding. Taking the field of oncology, where accelerated approval is the most prevalent, as an example, many accelerated approvals are shortcuts to overtake others by using response rate or progression-free survival as surrogate endpoints, rather than overall survival (OS) which has clear clinical significance. 87% of the drugs that have received accelerated approvals were marketed based on response rate as an endpoint.
While the use of surrogate endpoints is one of the key points of accelerated approval, the "conservative" use of the same endpoints in the post-marketing confirmatory research in an attempt to upgrade accelerated approval to full approval can be problematic. Given that surrogate endpoints may become disconnected from objective clinical benefits, FDA is likely to view the post-marketing research that disregard overall survival with a degree of discomfort. In fact, only 20% of confirmatory tests use overall survival as a research endpoint, leaving FDA with a sense of resignation, akin to "those who grasp my essence recognize my melancholy; those who do not comprehend me perceive a sense of yearning."
When clinically appropriate and logistically feasible, biopharmaceutical innovators should consider to provide overall survival data for the confirmatory tests after receiving accelerated approval.
Accelerated approval can sometimes be based on the single arm study, a point that is supported by data. Since the accelerated approval policy was introduced in 1992, approximately 72% of accelerated approvals relied on single arm studies. Clearly, single arm study lacks a control arm, which can potentially pose challenges in interpreting the clinical significance.
In addition to this, the use of single arm data can lead to an underestimation of benefits. Due to the lack of comparison of control groups, it is difficult to determine the true effect of treatment. In such cases, cost-benefit analysis often relies on models.
To strengthen study designs, especially given the rarity of certain diseases and the applicability of related strategies, one can consider increasing the use of randomized tests, avoiding single arm studies, and improving traditional small sample sizes. Randomized tests are effective in comparing the effects of different treatment methods because they can randomly assign participants to different treatment groups or control groups, reducing the potential for bias. Avoiding single arm studies can also enhance the credibility of the research. Improving traditional small sample sizes can increase the statistical power and reliability of the study.
The applicant, as a regulated party, also has the power to advise FDA to establish clear and specific regulations on key rules, rather than being vague or exceeding the scope of regulations. For example, the FDORA accelerated approval decision was made at the same time that the confirmatory research must be "in progress," leaving a lot of "room for misunderstanding," and FDA said that it was in the process of developing specific guidance to prevent any unintended consequences.
Applicants can adopt a "proactive" strategy during the accelerated approval application process to interact with FDA and participate in establishing new standards.
Accelerated approval is perhaps one of FDA's most controversial regulatory activities. From Aduhelm to Elevidys, opposition has never stopped. FDA originally had numerous accelerated approval cases that could support their wise decisions, but it seems that they are unable to counter the controversies that have been manufactured. Any bold innovation is inevitably accompanied by controversy and even mistakes. In the process of gradually refining the accelerated approval system, FDA is also seeking a strategy to return to the fundamentals, maximizing the benefits of this policy intended to benefit patients while minimizing its drawbacks. This approach aims to bring hope to patients in dire situations while also striving to prevent the reckless proliferation of ineffective drugs, akin to "charlatans talking about Zen".
Langreth, R. et al. Drug Companies Are Minting Billions on Unproven Treatments With FDA Shortcut. Bloomberg. 15. 05. 2023.
Rutherford, F. et al. FDA Hearing Targets Unproven $900,000 Drug for Deadly Cancer (1). Bloomberg Law. 16. 11. 2023.
Maclean, R. FDA accelerated approval: implications for treatment innovation in oncology. PharmaLive.com. 01. 04. 2024.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


