Nurah AliJune 13, 2025
Tag: Synthetic APIs , Natural APIs , biologics
Active Pharmaceutical Ingredients (APIs) are manufactured either through chemical synthesis or extracted from natural sources, including plants, animals, or microorganisms. Synthetic APIs, typically small-molecule drugs produced via organic chemistry, dominate the market comprising about 90% of all medicines.¹
Natural APIs, on the other hand, consist of complex compounds like proteins, peptides, and plant-derived chemicals, and are used in biologic drugs such as insulin and monoclonal antibodies. While less common, these biologics make up a significant portion of leading pharmaceuticals.²
Choosing between synthetic and natural APIs is a crucial decision for manufacturers, influencing product quality, production efficiency, cost, and regulatory requirements. Each route offers unique benefits and challenges—synthetic APIs provide consistency and scalability, while natural sources offer structural complexity and biocompatibility, but may suffer from variability and supply issues.
This article discusses the key differences between synthetic and natural APIs, focusing on how they affect regulation, production, and sustainability, to guide manufacturers in choosing the right approach.
· Synthetic APIsare typically highly pure, single-component substances. Manufactured under GMP conditions, they offer consistent quality with purity often exceeding 99%. Their defined molecular structures are easily confirmed using techniques like NMR or mass spectrometry, simplifying regulatory documentation.
· Unlike synthetic APIs, those obtained from natural sources are typically more intricate and challenging to fully characterize. Even after purification, they may contain trace impurities or variant forms. Large biologics, in particular, are defined more by their production process than by a fixed molecular structure. As a result, natural API manufacturers must use advanced analytical methods to identify active ingredients and monitor impurities.³
· Synthetic APIs offer high consistency across batches due to controlled chemical synthesis and in-line quality controls. Potency and impurity profiles remain stable, making replication straightforward.
· Natural APIs, however, are more variable. Factors like harvest season, geography, or microbial culture conditions can affect yield and composition. To manage this, manufacturers use strategies such as blending harvests, standardizing to marker compounds, or applying chromatographic profiling.⁴ Regulatory bodies also require clear evidence that each batch of a natural API matches the therapeutic profile of clinical trial materials—often demanding more rigorous controls than for synthetic APIs.
· Synthetic APIs are typically stable, with long shelf lives and minimal sensitivity to temperature or pH. Many can be stored at room temperature and easily formulated into tablets or capsules.
· Natural APIs, particularly biologics, are more delicate. Proteins and live biologics often require cold storage (2–8 °C), are sensitive to heat, light, or humidity, and may degrade over time. As a result, they often need special formulations—like freeze-drying and careful packaging to maintain stability.⁵ This makes natural APIs more complex and costly to store and transport compared to synthetic ones.
· Synthetic APIs, being small and stable, can be easily formulated into tablets, capsules, injectables, or patches. They’re generally compatible with many excipients and survive digestion, making oral delivery common.
· Natural APIs, especially biologics, are larger and more fragile. Many can't be taken orally due to degradation in the digestive tract and must be injected or infused. Some compounds need unique formulations, such as encapsulating oily extracts or using stabilizing buffers for proteins.⁶ Overall, synthetic APIs allow for more formulation versatility, whereas natural APIs often require customized delivery approaches
· Synthetic APIs are generally easier to scale. Once a chemical process is optimized, production can be increased using larger reactors or continuous manufacturing, independent of biological cycles. This flexibility proved vital during the COVID-19 pandemic when synthetic ingredients were rapidly stockpiled.
· Scaling natural APIs is more complex. Production often depends on biological materials such as plants or animals, which require time, land, and stable growing conditions. For example, early production of the cancer drug paclitaxel was limited by its source, the Pacific yew tree, until semi-synthetic and fermentation-based methods were developed.⁷
While synthetic processes scale predictably, natural APIs may need innovative solutions like biotech fermentation or engineered crops to meet demand.
· Once chemical methods are refined, producing synthetic APIs is generally more cost-effective. They benefit from low-cost raw materials, efficient scaling, and streamlined production, making drugs like aspirin and paracetamol highly affordable.
· Natural APIs, by contrast, typically involve higher costs. Extraction from plants or animals can be labor-intensive with low yields, and biologics made via fermentation or cell culture require expensive equipment and purification steps. For example, insulin was once sourced from animal pancreases, but recombinant DNA technology has enabled more efficient and cost-effective bacterial production.⁸
Overall, synthetic APIs tend to be more economical, while natural APIs and biologics often come with higher production costs due to complexity and processing demands.
· Synthetic APIs rely on chemical inputs that are widely available, stable in price, and easily sourced from global suppliers. This makes their supply chain generally reliable, with occasional risks from rare reagents or single-source materials.
· Natural APIs, however, depend on biological sources like plants or animals, which are subject to seasonal cycles, weather, disease, and geographic limitations. Farming or harvesting at scale can be costly and time-consuming, and wild-sourced materials may raise sustainability or regulatory concerns.For instance,Using pig intestines to produce heparin has led to concerns about supply stability, encouraging the search for synthetic alternatives.⁹
Overall, sourcing for natural APIs is more complex and prone to disruption, requiring careful supply chain planning and sometimes ethical or environmental safeguards.
· Synthetic APIs have streamlined logistics. Raw materials for synthetic drugs are usually shipped to centralized facilities, and the final products often remain stable at room temperature, simplifying both storage and transportation.
· Natural APIs involve more complex logistics. Agricultural materials must be harvested, transported, and processed quickly due to perishability. Biologics and vaccines often require cold chain storage throughout the supply chain, increasing cost and complexity. Additional regulations may also apply to biological materials, such as permits and phytosanitary checks.
Overall, synthetic APIs are easier and cheaper to distribute, while natural APIs require careful handling and temperature control from source to end use.
· Synthetic APIs are generally easier and faster to scale, benefiting from economies of scale and quick ramp-up through increased batch runs or reactor capacity. This allows for cost-effective production and rapid response to demand spikes, as seen during the COVID-19 pandemic.
· Natural APIs are harder to scale efficiently. Increasing output often means more land, labor, or biological material, which doesn't always reduce costs proportionally. Scaling can also be slower due to crop cycles or the time needed to grow production organisms.¹⁰ Advancements in biotechnology, such as fermentation techniques, have made it possible to produce natural compounds like insulin and monoclonal antibodies on a large scale, though this requires substantial investment.
Overall, synthetic routes offer more predictable and flexible scalability, while natural APIs may require strategic shifts or hybrid solutions to meet growing demand.
· Drug approval pathways vary depending on whether the active pharmaceutical ingredient (API) is synthetic or naturally derived. Small-molecule synthetic drugs typically follow the standard New Drug Application (NDA) process in the U.S., focusing on well-defined quality, safety, and efficacy. Generics can use an abbreviated pathway based on bioequivalence, avoiding full clinical trials.
· In contrast, biologics (large, complex molecules like proteins or vaccines) are approved through the Biologics License Application (BLA), which emphasizes consistent manufacturing due to inherent variability. Biosimilars—biologic equivalents to generics require extensive data, including clinical studies, to demonstrate similarity without meaningful differences.
· Natural mixtures and botanical drugs face unique challenges. The FDA’s Botanical Drug Development guidance requires robust characterization and reproducibility, often supported by chemical profiling and clinical evidence. Similarly, the European Medicines Agency (EMA) offers frameworks for herbal products, allowing traditional use registrations in specific cases.¹¹
Overall, while synthetic APIs benefit from established standards and streamlined processes, biologics and natural products demand more rigorous Chemistry, Manufacturing, and Controls (CMC) documentation and post-market surveillance due to complexity and variability.
· All drug types require extensive documentation, but synthetic APIs benefit from standardized frameworks like USP or EP monographs, which define purity, identity, and dissolution criteria. Their well-defined structures and processes allow consistent manufacturing with relatively straightforward validation.
· In contrast, natural APIs lack inherent uniformity, so manufacturers must define and justify their own specifications. For botanical products, this includes documenting the source plant, processing methods, and ensuring batch consistency using marker compounds and fingerprint chromatograms. Reference materials and clinical consistency may also be required if active constituents aren't fully identified.
· Biologic drugs are subject to the highest level of regulatory scrutiny, requiring comprehensive records of the cell source, growth environment, and purification methods, as the manufacturing process directly influences the final product. Removing contaminants and ensuring viral safety must be validated, and even small process modifications can trigger the need for additional studies. After approval, these products also undergo strict ongoing evaluations to ensure long-term stability.
Although standards for biologics and herbal products are gradually expanding, they are still not as comprehensive. Therefore, manufacturers working with these APIs must provide extensive documentation and implement strict quality control measures to satisfy regulatory demands.
· Environmental concerns are increasingly important in pharmaceutical manufacturing. Synthetic APIs often involve hazardous chemicals, generate waste, and consume energy, prompting stricter regulatory oversight. In response, companies are adopting green chemistry—using safer solvents, improving atom economy, and reducing toxic by-products—to lower waste and environmental costs.
· Natural APIs face different sustainability challenges. Harvesting plants at scale can cause habitat loss, pesticide overuse, or species depletion. To address this, regulators and industry initiatives promote sustainable practices like cultivation under Good Agricultural and Collection Practices (GACP). Some species are protected by international agreements (e.g., CITES), requiring proof of legal or cultivated sourcing.
· Animal-derived products also raise environmental and ethical issues, pushing companies toward synthetic or biotech alternatives—like plant cell fermentation for paclitaxel or recombinant DNA methods for proteins. Environmental Risk Assessments (ERAs) are required in many jurisdictions, focusing on a drug's ecological footprint, such as residue release into waterways.
In sum, while synthetic APIs must tackle pollution and energy use, natural APIs must ensure sustainable sourcing and biodiversity protection. Regulatory and corporate priorities now emphasize minimizing environmental impact across all API types.
Table: The key distinctions between synthetic and natural APIs.
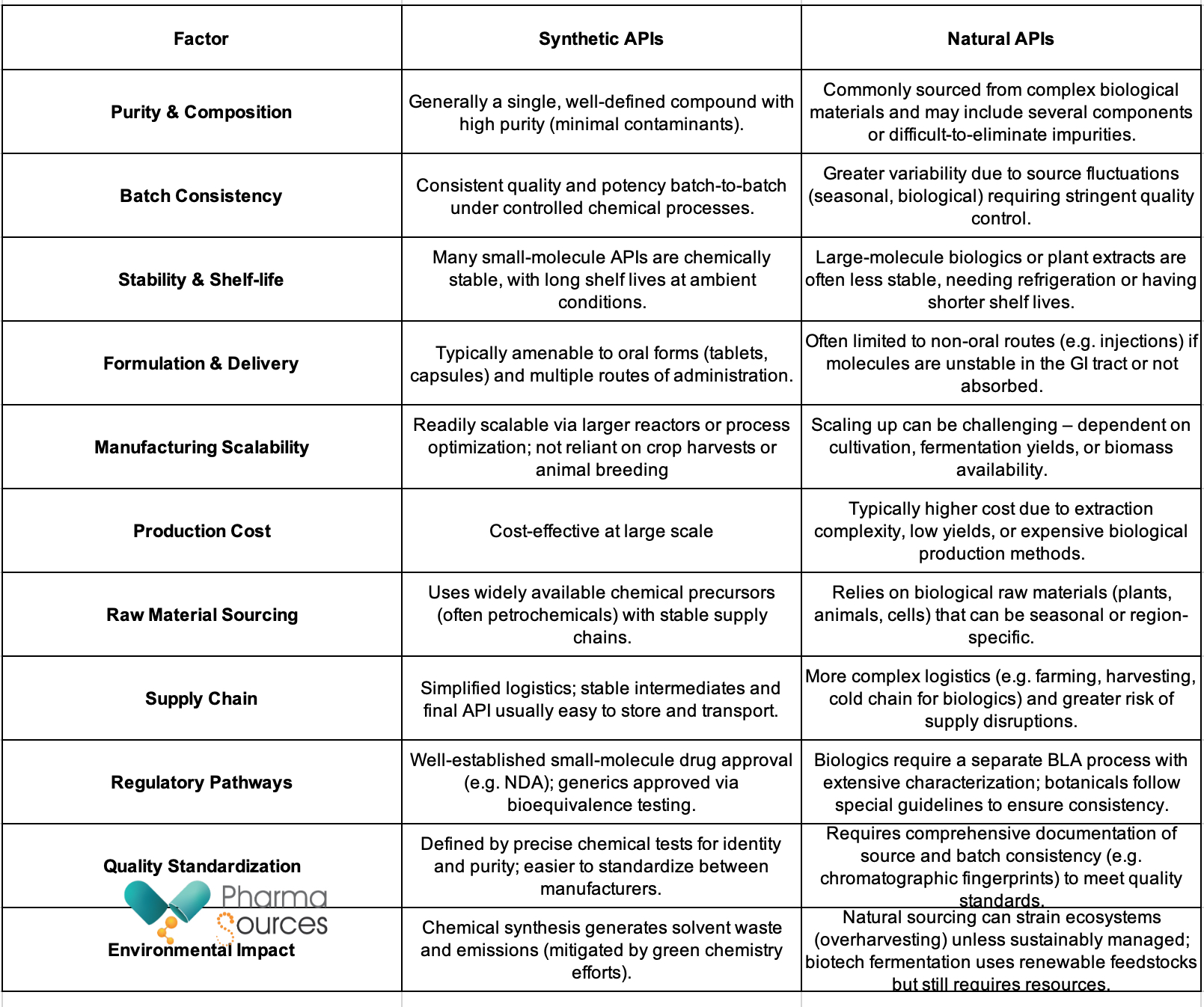
Both synthetic and natural APIs are essential in drug development. The right choice depends on the drug’s properties, regulatory requirements, company capabilities, and market strategy.
· Drug Characteristics: Small, stable molecules are typically better suited for chemical synthesis, while complex biologics (e.g., antibodies, enzymes) require natural or biotechnological methods.
· Quality and Formulation: Synthetic APIs offer purity and control, ideal for drugs requiring tight impurity limits. Natural APIs may be preferred when efficacy relies on a mix of active compounds, though they require rigorous standardization.
· Operational Fit: Companies should align production methods with internal strengths—organic synthesis for firms with chemical expertise, and natural sourcing or bioprocessing for those with biotech or agricultural capacity.
· Cost and Market Positioning: Synthetic routes can lower costs and extend patent life. Natural APIs may appeal to “naturally derived” markets. Manufacturers should also consider speed to market and supply chain stability.
Many firms use hybrid strategies starting with natural sources and transitioning to synthetic or semi-synthetic production as technology and scalability improve (e.g., paclitaxel, artemisinin).
Bottom line: A flexible, evidence-based approach evaluating technical feasibility, regulatory needs, and economic factors enables manufacturers to deliver safe, effective, and sustainable medicines while staying competitive.
1.Simić, S., Zukić, E., Schmermund, L., Faber, K., Winkler, C. K., & Kroutil, W. (2021). Shortening Synthetic Routes to Small Molecule Active Pharmaceutical Ingredients Employing Biocatalytic Methods. Chemical Reviews, 122(1), 1052–1126. https://doi.org/10.1021/acs.chemrev.1c00574
2.Nicholas, J. M. (2012). Complex Drugs and Biologics: Scientific and Regulatory Challenges for Follow-on Products. Drug Information Journal, 46(2), 197–206. https://doi.org/10.1177/0092861512437759
3.rommelin, D. J. A., Shah, V. P., Klebovich, I., McNeil, S. E., Weinstein, V., Flühmann, B., Mühlebach, S., & de Vlieger, J. S. B. (2015). The similarity question for biologicals and non-biological complex drugs. European Journal of Pharmaceutical Sciences, 76, 10–17. https://doi.org/10.1016/j.ejps.2015.04.010
4.Xiong, H., Yu, L. X., & Qu, H. (2013). Batch-to-Batch Quality Consistency Evaluation of Botanical Drug Products Using Multivariate Statistical Analysis of the Chromatographic Fingerprint. AAPS PharmSciTech, 14(2), 802–810. https://doi.org/10.1208/s12249-013-9966-9
5.Yu, Y. B., Briggs, K. T., Taraban, M. B., Brinson, R. G., & Marino, J. P. (2021). Grand Challenges in Pharmaceutical Research Series: Ridding the Cold Chain for Biologics. Pharmaceutical Research, 38(1), 3–7. https://doi.org/10.1007/s11095-021-03008-w
6.Mitragotri, S., Burke, P. A., & Langer, R. (2014). Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nature Reviews Drug Discovery, 13(9), 655–672. https://doi.org/10.1038/nrd4363
7.Quave, C. (2024). The Magic of Nature’s Pharmacy: Lessons from the Yew Tree | Emory University | Atlanta GA. Emory.edu. https://winshipcancer.emory.edu/magazine/issues/2024/spring/in-the-news/pioneering-perspective/index.html
8.Sandow, J., Landgraf, W., Becker, R., & Seipke, G. (2015). Equivalent Recombinant Human Insulin Preparations and their Place in Therapy. European Endocrinology, 11(1), 10. https://doi.org/10.17925/ee.2015.11.01.10
9.Chandarajoti, K., Liu, J., & Pawlinski, R. (2016). The design and synthesis of new synthetic low-molecular-weight heparins. Journal of Thrombosis and Haemostasis, 14(6), 1135–1145. https://doi.org/10.1111/jth.13312
10.magazine, M. P. (n.d.). Synthetic Biology’s First Malaria Drug Meets Market Resistance. Scientific American. https://www.scientificamerican.com/article/synthetic-biology-s-first-malaria-drug-meets-market-resistance/
11.Gherghescu, I., & Delgado-Charro, M. B. (2020). The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA an


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


