David Orchard-WebbJuly 08, 2025
Tag: Trastuzumab Biosimilars Sales , Atorvastatin Global Demand , Rituximab Biosimilar Adoption , Levothyroxine Prescription Trends , Bevacizumab Oncology Biosimilars
The global pharmaceutical market is approaching a phase in which several high-revenue medicines will lose patent protection, paving the way for generic and biosimilar competition (Scheckel, 2021). According to industry estimates, around 55 biologic blockbusters, each worth over $1 billion in peak sales (potentially $270 billion in total), will go off-patent by 2030 (Grover, 2022). This "patent cliff" implies that some of the top-selling pharmaceuticals in 2025 will most likely be copies of the best-sellers from the previous decade. Generic and biosimilar entrants often undercut original price (sometimes by 80-90%), making them appealing to payers and governments (Grover, 2022). In this article we anticipate the top five selling generic pharmaceuticals in 2025, including biosimilars, to be trastuzumab, Atorvastatin generics rituximab, Levothyroxine generics, and bevacizumab. These therapies address the primary areas of medical need in oncology, including breast, gastric, colorectal, lung, and renal cancers; B-cell malignancies and autoimmune diseases; hypothyroidism and thyroid cancer; and hyperlipidemia management and cardiovascular disease prevention.
These predictions are based on sales data and market size predictions. The forecasts are based on prescription patterns in the United States and global market estimations, with the understanding that specific global sales statistics can be difficult to obtain owing to varied market dynamics, and that all projections are forward-looking based on recent trends.
Trastuzumab, is a monoclonal antibody originally invented by Roche (Genentech, Inc.) under the brand Herceptin®, which was worth $6 - 7.5 billion before patent expiry (GaBI, 2018), which changed the landscape of oncotherapy for HER2-positive breast and gastric cancer. With the originator's patents expiring in major markets between 2014 and 2019 (Queen, 1996) (Hudziak, 2002), a variety of US FDA approved biosimilars have emerged, including Pfizer's Trazimera®, Celltrion's Herzuma®, Mylan's Ogivri, Organon’s Ontruzant, Amgen’s Kanjinti, and Accord’s Hercessi (FDA, 2025b). In Q3 2024, 86% of the US trastuzumab market share was captured by biosimilars, the rest being Herceptin® (Samsung, 2025). In Europe biosimilar market coverage is even greater (Grover, 2022).
Trastuzumab biosimilars are expected to produce more than $5.5 billion in worldwide sales by 2025 (TBRCb, 2025), with rapid biosimilar market expansion in the Asia-Pacific (APAC), Europe, and to a lesser extent, the Middle East and Africa (MEA), in addition to the United States. South America lags behind with 5% of the worldwide trastuzumab biosimilars (Tables 1-5) (VMR, n.d.). Within the three largest markets of the APAC region (China, India, and Japan) eight trastuzumab biosimilars have been approved. Competitive advantages of biosimilars include substantially reduced cost (typically 20-50% savings) and easy interchangeability, which should put trastuzumab biosimilars firmly at the top of the 2025 sales rankings.
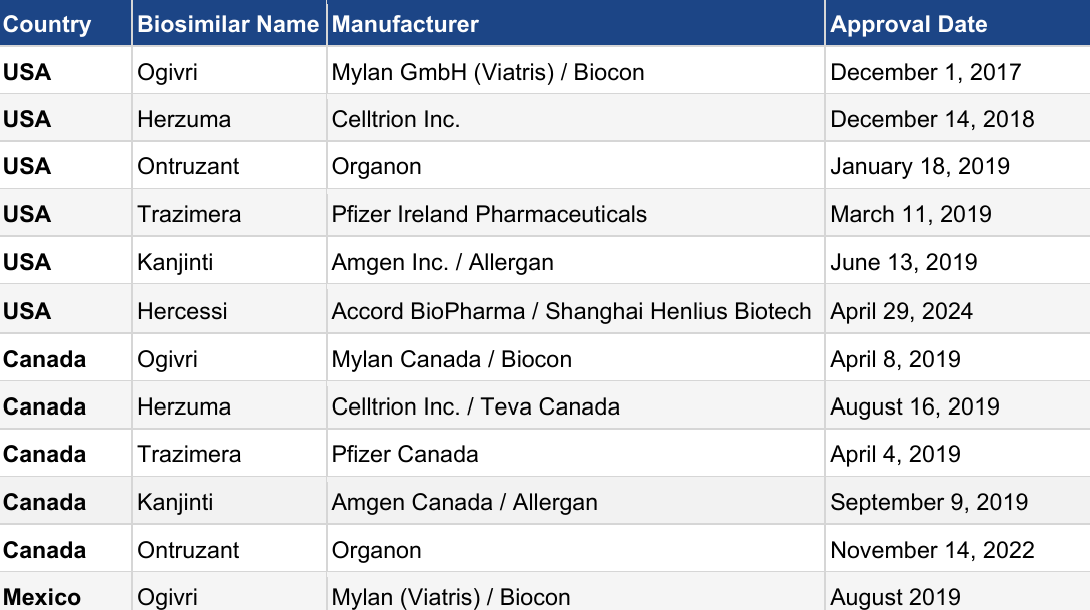
Table 1: Trastuzumab biosimilars approved in North American markets
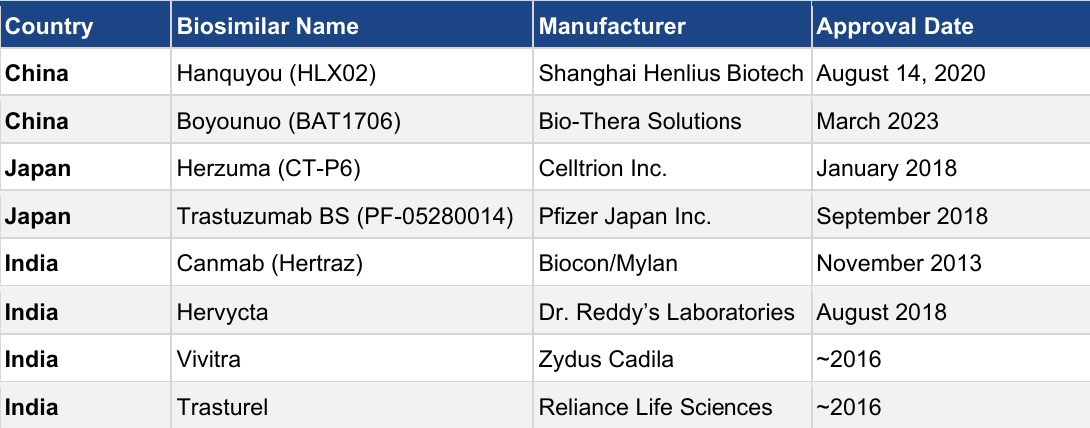
Table 2: Trastuzumab biosimilars approved in the top three APAC markets
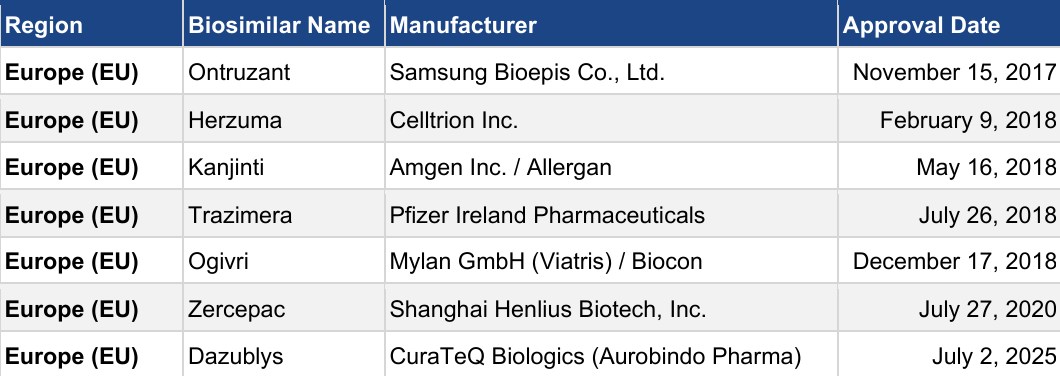
Table 3: Trastuzumab biosimilars approved in European markets
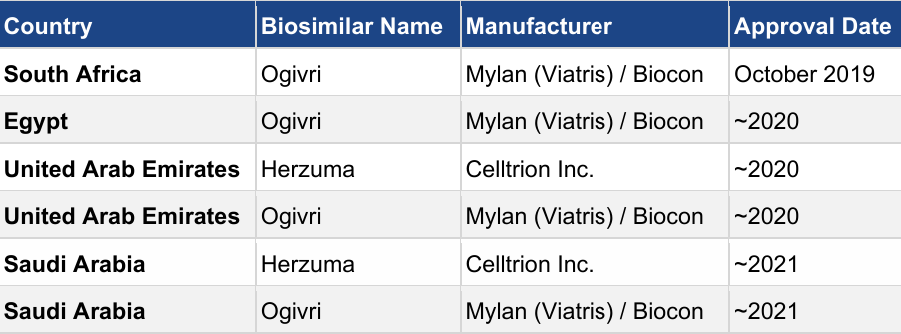
Table 4: Trastuzumab biosimilars approved in Middle East and African (MEA) markets
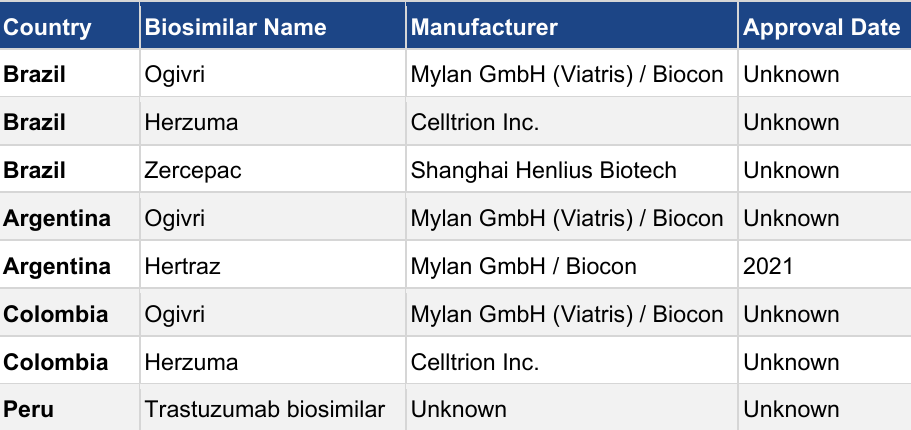
Table 5: Trastuzumab biosimilars approved in South American markets
Over a decade after the Lipitor® brand lost exclusivity, atorvastatin, a frequently prescribed statin for dyslipidemia and cardiovascular risk reduction, is still a staple in generic pharma markets (Mikulic, 2024). Its global success is attributed to massive patient demand, guideline-based first-line usage, and price-driven uptake in both high-income and emerging nations (Guadamuz, 2022).
Atorvastatin generics are estimated to earn $3.6 billion in global sales by 2025 (GPR, 2023). CMP Development LLC, Accord Healthcare Inc., Agnitio Inc., Alkem Laboratories Ltd, and Apotex Inc., to name a very limited selection, manufacture atorvastatin (FDA, 2025a), and its low cost has made it the world's most popular statin by volume (Patel, 2019). Competitive benefits include long-term safety, widespread payer coverage, and inclusion on practically every national essential medications list. Market adoption is nearly ubiquitous in public health systems, ranging from the Veterans Affairs formulary in the United States to India's Jan Aushadhi scheme (IoM, 2000).
Rituximab, formerly marketed as Rituxan® by Roche and Biogen, is a monoclonal antibody that targets CD20 and is commonly used to treat non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis. Rituxan (rituximab) biosimilars. Rituximab is a monoclonal antibody (anti-CD20) that treats non-Hodgkin lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis. Its biosimilars (e.g., Amgen's Riabni, Pfizer's Ruxience, Teva's Truxima, and Sandoz's Riximyo) are widely known worldwide (FDA, 2025b). The biosimilar market for rituximab is expected to increase from ~$3.03 billion in 2024 to about $3.47 billion in 2025 (CAGR ~15%) (TBRCa, 2025). Biosimilars are expected to account for approximately 76% of rituximab volume in the United States by late 2024 (Samsung, 2025), and are also widely used in Europe (Grover, 2022). Rituximab's broad indication (various cancer and immunological illnesses) and validated clinical profile result in significant patient volumes, which biosimilars exploit by cutting prices. Europe and North America have had substantial penetration, but Asia-Pacific regions also contribute significantly.
Levothyroxine, a generic synthetic form of the thyroid hormone T4, is still one of the most widely prescribed drugs for hypothyroidism and thyroid cancer (Virili, 2018). As a small-molecule generic, its long-term supremacy stems from consistent demand, large patient populations, and inexpensive production costs.
The global oral levothyroxine sodium market is expected to be worth $2.5–3.0 billion by 2025 (DIM, n.d.). This market is supplied by a number of firms, including AbbVie (Synthroid®), Fresenius Kabi USA Llc, Maia Pharmaceuticals Inc., Onesource Specialty Pte Ltd, Piramal Critical Care Ltd, Xiromed Pharma Espana Sl, and Zydus Pharmaceuticals USA Inc. (FDA, 2025a). Despite its simple pharmacology, levothyroxine needs strict bioequivalence requirements due to its limited therapeutic index. Regulatory monitoring in places such as the United States, where levothyroxine generics must demonstrate strict equivalence, has not impeded their market penetration. Generic levothyroxine has the highest prescription volume in North America (Mikulic, 2024), Europe, and Latin America, demonstrating both clinical reliance and cost-driven legislative incentives.
Bevacizumab is an anti-angiogenic antibody used to treat colorectal, lung, kidney, and other malignancies. Biosimilars, such as Amneal's Alymsys, Amgen's Mvasi, Pfizer's Zirabev, and Organon's Aybintio, have been available from 2019-2020. Analysts expect bevacizumab biosimilar revenues to increase from around $1.53 billion in 2024 to $1.66 billion in 2025 (TBRCc, 2025). Supportive regulatory regulations led to quick acceptance of biosimilars throughout Europe, with certain regions accounting for around 90% of the market (Grover, 2022). Biosimilars accounted for approximately 64% of total volume in the United States by late 2024 (Samsung, 2025), although adoption is increasing with new releases. The increased number of cancer indications and infusions in hospitals results in high volume consumption. As oncology costs tighten, payers are shifting aggressively to bevacizumab biosimilars. The significant growth of this class and the presence of many worldwide companies place bevacizumab copies among the top generics (TBRCc, 2025).
In conclusion, the expected top five selling generics and biosimilars for 2025 illustrate global healthcare's strategy change toward cost-effective, high-impact medicines in areas with sustained clinical need. Trastuzumab biosimilars are expected to lead worldwide sales, owing to extensive oncology usage and strong adoption in the United States, Europe, and Asia-Pacific. Because of its importance in lowering cardiovascular risk, atorvastatin generics continue to be in high demand across the world. Rituximab biosimilars continue to gain traction in cancer and immunology markets, particularly in developed countries. Levothyroxine generics remain crucial for thyroid problems, as evidenced by stable global prescription volumes. Finally, bevacizumab biosimilars are quickly developing in cancer markets, driven by increased pricing constraints and payer incentives. Together, these goods demonstrate how patent expirations and changing healthcare economics are transforming the global pharmaceutical landscape, with biosimilars and established generics boosting market value and access to critical therapies.
DIM - Data Insights Market. (n.d.). Oral levothyroxine sodium report [Market report]. Retrieved July 6, 2025, from https://www.datainsightsmarket.com/reports/levothyroxine-sodium-1164586#summary
FDA. (2025a). Orange Book: Approved drug products with therapeutic equivalence evaluations. U.S. Department of Health and Human Services. Retrieved July 6, 2025, from https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm
FDA. (2025b). Purple book: Database of FDA-licensed biological products with reference product exclusivity and biosimilarity or interchangeability evaluations. U.S. Department of Health and Human Services. Retrieved July 5, 2025, from https://purplebooksearch.fda.gov
GaBI. (2018, June 21). Biosimilars of trastuzumab. GaBI Online. Retrieved July 5, 2025, from https://www.gabionline.net/biosimilars/general/Biosimilars-of-trastuzumab
GPR - Growth Plus Reports. (2023, June 19). Atorvastatin drug market size to surpass US$ 6.05 billion by 2031. Yahoo Finance. Retrieved July 6, 2025, from https://finance.yahoo.com/news/atorvastatin-drug-market-size-surpass-133000208.html
Grover, N., & Scheer, S. (2022, November 22). Focus: Generic drugmakers Teva and Sandoz make major push to biosimilars. Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/generic-drugmakers-teva-sandoz-make-major-push-biosimilars-2022-11-22/
Guadamuz, J. S., Shooshtari, A., & Qato, D. M. (2022). Global, regional and national trends in statin utilisation in high-income and low/middle-income countries, 2015–2020. BMJ Open, 12(9), e061350. https://doi.org/10.1136/bmjopen-2022-061350
Hudziak, R. M., Shepard, H. M., Ullrich, A., & Fendly, B. M. (2002). Monoclonal antibodies directed to the HER2 receptor (U.S. Patent No. 6,399,063 B1). United States Patent and Samsung Bioepis. (2025). Biosimilar market dynamics: 8th edition, Q1 2025. Samsung Bioepis Co., Ltd. https://www.samsungbioepis.com
IoM - Institute of Medicine (US) VA Pharmacy Formulary Analysis Committee. (2000). The VA national formulary and veterans health care. In D. Blumenthal & R. Herdman (Eds.), Description and analysis of the VA national formulary (Chapter 6). National Academies Press (US). Retrieved July 6, 2025, from https://www.ncbi.nlm.nih.gov/books/NBK225695/
Mikulic, M. (2024, November 25). Top generic drugs in the U.S. by dispensed prescriptions 2023. Statista. Retrieved July 6, 2025, from https://www.statista.com/statistics/869618/top-unbranded-generic-drugs-by-prescriptions-dispensed-in-us/
Patel, N., Bhargava, A., Kalra, R., Parcha, V., Arora, G., Muntner, P., & Arora, P. (2019). Trends in lipid, lipoproteins, and statin use among U.S. adults. Journal of the American College of Cardiology, 74(20), 2525–2528. https://doi.org/10.1016/j.jacc.2019.09.026
Queen, C. L., & Selick, H. E. (Inventors); Protein Design Labs, Inc. (Assignee). (1996). Humanized immunoglobulins and their production and use (European Patent No. EP 0451216 B1). European Patent Office. Retrieved July 5, 2025, from https://patents.google.com/patent/EP0451216B1/en
Samsung Bioepis Co., Ltd. (2025). SB biosimilar market report Q1 2025: 8th edition. Samsung Bioepis. Retrieved July 6, 2025, from https://www.samsungbioepis.com/en/biosimilar-market-report
Scheckel, C. J., & Rajkumar, S. V. (2021). Generics and biosimilars: Barriers and opportunities. Mayo Clinic Proceedings, 96(12), 2947–2957. https://doi.org/10.1016/j.mayocp.2021.08.001
TBRCa - The Business Research Company. (2025). Rituximab biosimilar global market report 2025: Size, share & trends. The Business Research Company. Retrieved July 6, 2025, from https://www.thebusinessresearchcompany.com/report/rituximab-biosimilar-global-market-report#:~:text=The%20rituximab%20biosimilars%20market%20size,biosimilars%2C%20and%20emerging%20markets%20growth
TBRCb. (2025, January). Trastuzumab biosimilar global market report 2025: Size, share & forecast 2024–2029. The Business Research Company. https://www.thebusinessresearchcompany.com/report/trastuzumab-biosimilar-global-market-report
TBRCc (2025). Bevacizumab biosimilars global market report 2025: Size, share & trends. The Business Research Company. Retrieved July 6, 2025, from https://www.thebusinessresearchcompany.com/report/bevacizumab-biosimilars-global-market-report#:~:text=The%20bevacizumab%20biosimilars%20market%20size,access%20and%20affordability%2C%20biosimilar%20acceptance
Virili, C., Giovanella, L., Fallahi, P., Antonelli, A., Santaguida, M. G., Centanni, M., & Trimboli, P. (2018). Levothyroxine therapy: Changes of TSH levels by switching patients from tablet to liquid formulation. A systematic review and meta-analysis. Frontiers in Endocrinology, 9, Article 10. https://doi.org/10.3389/fendo.2018.00010
VMR - Verified Market Reports. (n.d.). Trastuzumab biosimilar market. Retrieved July 6, 2025, from https://www.verifiedmarketreports.com/product/trastuzumab-biosimilar-market/?utm_source=chatgpt.com/
Industry forecasts and market reports were used throughout. Key sources include global sales forecasts for biosimilarsthebusinessresearchcompany.comthebusinessresearchcompany.comthebusinessresearchcompany.comcoherentmarketinsights.comcoherentmarketinsights.com and analyses of market share trendsajmc.comajmc.comreuters.comreuters.comreuters.com. Specific references: peer-reviewed and trade publications (AJMC, Reuters), as well as market research releases by Coherent Market Insights, The Business Research Company, and Exactitude/GlobeNewswire, among others, are cited above. Each citation corresponds to a connected source used to compile this report.
David Orchard-Webb, Ph.D., is a technical writer with broad interests including health & technology writing, plus extensive training and knowledge of biomedicine and microbiology. My Ph.D. and postdoc were in oncology and developing cancer medicines. I provide technical medical and other writing services for projects ranging from “knowledge automation” to pure pharma, to food safety, to the history of science, and everything in between. I also provide white papers, ebooks, meta-analysis reviews, editing, consulting, business, and market research-related activities in biomedicine, technology, and health. In addition to its well-known role in the development of medicines, I am a big believer in biotechnology’s ability to revolutionize industries such as food-tech, agtech, textiles & fashion.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


