Saher HaiderSeptember 19, 2025
Tag: Digitalization , AI , Blockchain
Over the last couple of years, AI and blockchain have made inroads into the life sciences industry, including the pharmaceutical supply chain network. What was once considered a tedious task spanning multiple departments and involving countless stakeholders is now on the brink of complete automation.
However, AI and blockchain technologies go far beyond improving the efficiency of procurement operations. With stricter regulatory requirements, global supply disruptions, and the growing threat of counterfeit drugs making their way to the market, digitalization is becoming a strategic necessity.
Digitalization in pharma procurement combines AI-driven demand forecasting, blockchain-based traceability, IoT-enabled monitoring, and workflow automation to create a transparent, reliable, and compliant procurement process in the pharmaceutical sector.
In this article, we will discuss exactly that. We’ll start off by exploring how AI forecasting and blockchain traceability are transforming pharmaceutical procurement. We will also examine the benefits of adapting these technologies for both buyers and suppliers, the challenges associated with adoption, and the best practices for fully embracing this digital transformation without compromising compliance.
One of the most persistent problems in pharmaceutical procurement is poor demand forecasting. And there’s considerable data to back this up:
Studies show that the supply chain network of pharma companies often struggles with inaccurate forecasting, long lead times, stockouts, overstocking, and staggering supply chain costs.
In another study conducted on Ethiopia’s public pharmaceutical supply system, researchers found that procurement delays were compounded by inaccurate forecasts due to workforce shortage and weak data quality.
AI-powered demand forecasting refers to the utilization of machine learning algorithms to study historical data, incorporating real-time data to make precise predictions, and adapting to changing market conditions through continuous learning. For example, a 2022 study on machine learning in pharma supply chains reported that advanced forecasting models can improve accuracy by 10% to 41%, reducing costly mismatches between supply and demand.
Another challenge that the pharma procurement system faces is the widespread prevalence of counterfeit and falsified medicines. According to the World Health Organization (WHO),1 in every 10 medical products in low- and middle-income countries are substandard or falsified, costing health systems over US$30 billion annually.
And this is where foolproof traceability systems take the plunge: blockchain traceability systems are filling this void by providing immutable, end-to-end visibility of every drug’s origin, movement, and handling.
Pharmaceutical procurement operates under some of the strictest regulatory requirements in the world, yet many systems lack the traceability needed for full compliance. Blockchain technology provides tamper-proof audit trails and enables compliance with serialization laws and Good Distribution Practices. Once this tech is fully adopted, procurement teams will no longer have to deal with fragmented data systems, manual paperwork, and difficulties in proving regulatory adherence across borders.
Drug shortages remain a visible symptom of weak procurement systems, causing shortages in around 10% of essential drugs with potential reductions to 4-5% if supply chain resilience improves.
Apart from supply chain disruptions, compromised procurement systems also struggle with cold chain management. This is again where AI forecasting and IoT come in handy:
AI forecasting can help mitigate drug shortages by predicting supply–demand mismatches way early, while blockchain and IoT integration can enable procurement teams to monitor temperature-sensitive products in real-time throughout the supply chain.
As discussed already, traditional demand forecasting methods in the pharma industry have their own limitations in maintaining target inventory. The systems cease to perform when demand is intermittent, promotion-sensitive, or seasonally volatile. AI-based systems solve these issues by reducing stockouts and write-offs.
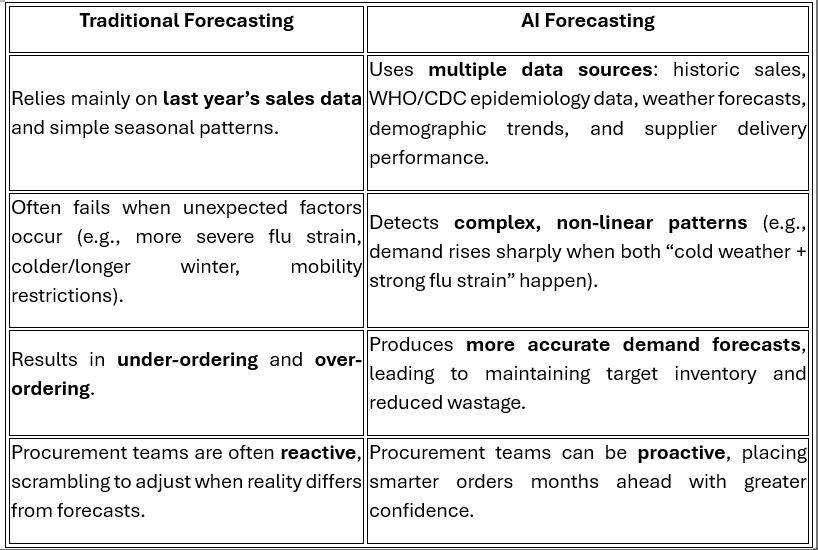
Unlike traditional forecasting methods, ML models use multiple data sources and spot complicated patterns, which procurement teams use to keep just the right amount of inventory in stock.
The data sources used by these ML models include:
· Historic sales data (data from previous sales)
· Therapy trends (which drug classes are in demand)
· Marketing calendars (when promotions or product launches happen).
· Epidemiology data (disease outbreaks that drive demand).
· Lead-time variability (how supplier delivery times fluctuate).
· External data like weather (flu season linked to cold weather) or mobility (pandemic restrictions).
Let’s take a look at the following example of a flu vaccine procurement method, comparing traditional forecasting with AI-powered forecasting:
Apart from demand forecasting, ML models can also make the entire procurement procedure for pharma companies pain-free by filtering out which suppliers are reliable and how long deliveries take.
For example, one study predicted delivery times with less than a 2-day error. ML can also score suppliers based on past reliability, quality, and compliance. Thus, using ML-based insights, companies can choose better suppliers and plan purchases more accurately, avoiding late deliveries or expensive last-minute fixes.
In pharma procurement, traditional systems of traceability rely on centralized databases that often lack visibility across the entire supply chain and are vulnerable to tampering.
This is where the deployment of blockchain tech comes in.
Blockchain introduces a decentralized, tamper-evident ledger where each stakeholder records standardized supply chain events such as packaging, shipping, receiving, and decommissioning. It provides a “single source of truth” through which every transaction is verified and time-stamped, making it challenging for counterfeit or diverted products to infiltrate legitimate supply chains.
Pilot projects under the U.S. Drug Supply Chain Security Act (DSCSA) and peer-reviewed studies confirm that blockchain can address persistent visibility gaps, provided that challenges of interoperability, scalability, and data privacy are managed effectively.
Counterfeit and falsified medicines are costing health systems billions of dollars annually and placing patients at serious risk. To address this risk, regulators have mandated serialization, which is assigning a unique identifier to every saleable unit of medicine.
In the European Union, the Falsified Medicines Directive (FMD) requires each package to carry a unique identifier and an anti-tampering device. These identifiers are uploaded into national repositories linked to the European Medicines Verification System (EMVS), where pharmacies can verify authenticity before dispensing.
Pharmaceutical supply chains are governed by strict global regulations, and blockchain is increasingly being tested as a tool to support compliance.
The DSCSA in the US requires interoperable, electronic tracing of drugs at the package level across all authorized trading partners. While blockchain is not mandated, pilot studies show it can meet the act’s requirements for secure, real-time information sharing and verification.
In the EU, compliance focuses on unique identifiers and anti-tampering devices, with verification carried out through the EMVS. Blockchain can complement this system by enhancing transparency and auditability.
Successful adoption of blockchain tech requires integration with existing ERP and warehouse management systems, protection of sensitive business data, and alignment with GS1 standards to ensure interoperability. Governance questions, such as who operates the nodes and how disputes are resolved, must also be addressed.
Temperature-sensitive pharmaceutical products such as vaccines, biologics, and cell and gene therapies require specific storage and handling conditions throughout the pharma supply chain. However, it is easier said than done. Failures in cold chain management are estimated to cost the biopharma industry billions of dollars annually, with the main reason being spoilage from temperature excursions.
Fortunately, IoT (Internet of Things) sensors are now solving this problem. These are devices that are being deployed in modern cold chain monitoring systems to continuously track temperature, humidity, vibration, and location throughout transport and storage. What makes this tech work is that the data from these sensors can be transmitted in real time to centralized dashboards, enabling procurement and quality teams to:
• Intervene immediately when temperature excursions occur.
• Verify compliance with GDP requirements for storage and transport.
• Improve forecasting inputs, as AI models can learn from environmental and logistical data to refine lead-time and spoilage risk predictions.
When integrated with blockchain, IoT sensor data gains additional value. It enables the storage of every temperature reading or location, providing regulators and buyers with evidence that products were handled correctly.
The ‘traditional’ methods of procurement in pharmaceuticals are based on extensive manual effort. From purchase orders, supplier verifications, and payment releases to compliance checks, each step introduces delays and risks of human error.
With AI, blockchain, and IoT, most of these processes can be automated. Converging these technologies and then integrating them can transform the entire pharma procurement cycle from a reactive process into a predictive, autonomous system. It benefits buyers by minimizing endless manual reconciliations, suppliers by making their payments faster, and regulators by providing them with more reliable documentation trails.
AI forecasting, blockchain traceability, and IoT monitoring are transforming pharma procurement from a reactive process into one that is predictive, transparent, and automated. Together, they reduce shortages, prevent counterfeits, streamline compliance, and build trust between buyers and suppliers.
To stay competitive and resilient, procurement leaders must embrace these digital tools now. Explore trusted, innovation-ready suppliers on Pharmasources.com to begin building smarter, safer supply chains.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


