July 28, 2025
Tag:
I. Preface
Compound fertilizers refer to chemical fertilizers containing two or more nutrients. They have the advantages of high nutrient content, few by-products and good physical properties. They play a very important role in balanced fertilization, improving fertilizer utilization rate and promoting high and stable crop yields. However, during the production process of chemical fertilizers, some manufacturers, considering cost issues, choose ammonium chloride and potassium chloride as raw materials and thereby introduce chloride ions. Crops require very little chloride ions. Excessive chloride ions can affect seed germination and cause soil compaction and salinization, which is not conducive to agricultural production.
This method utilizes the principle that silver nitrate titrates chloride ions to form precipitates. It involves boiling the fertilizer in water, filtering it, and then directly titrating it with silver nitrate. The operation is simple, the titration speed is fast, the data repeatability is good, and the accuracy is greatly improved compared to the manual titration method. It can quickly provide a reliable basis for the detection of chloride ions.
Ii. Instruments and Reagents
2.1 Instruments
JH-T7 fully automatic titrator, silver ion selective electrode, electric ceramic furnace, analytical balance, etc
2.2. Reagents
Nitric acid (analytical grade), silver nitrate standard solution (0.1mol/L), purified water.
Iii. Experimental Methods
3.1 Analysis Steps
Accurately weigh 5.0g of the sample into a 250ml beaker, add 50ml of pure water to dissolve it, stir evenly, heat and boil for 10 minutes, cool, then make up to 250ml in a volumetric flask, dry filter, discard the initial part of the filtrate, and collect the filtrate for future use.
Adjust the instrument parameters properly, accurately draw 10ml of filtrate, add 50ml of pure water, titrate to the endpoint with 0.1mol/L silver nitrate standard solution, and record the consumed volume. At the same time, conduct blank experiments.
The parameters of the titrator are set as shown in Table 1
Table 1 Titration Parameter Settings
| Titration type |
Dynamic titration |
Method Name:: |
Determination of chloride content |
| Burette volume |
10mL |
Sample measurement unit: |
g |
| Working electrode | Composite silver electrode | Reference electrode | no |
3. Test graph example
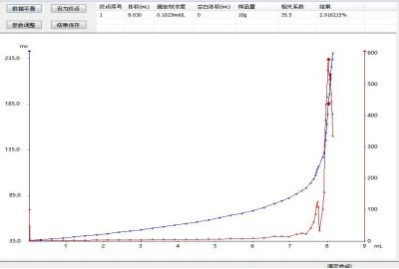
Iv. Results and Discussion
4.1 Experimental Results
The experimental results are shown in Table 2
Table 2 Test Results
|
Sample |
sample number |
sample quality/ml |
volumetric solution concentration (nitric acid silver)/(mol/L) |
the content of chlorine ion titration |
form-factor/ml (%) |
|
|
Blank |
/ |
0.1023 |
0.001 |
/ |
|
3# |
1 |
|
8.071 |
14.57 |
|
2 |
8.030 |
14.50 |
||
|
3 |
8.052 |
14.54 |
4.2. Conclusion
The determination of chloride ions in compound fertilizers by fully automatic potentiometric titration is rapid and has good data repeatability. It reduces the interference of indicator color on the results and improves the detection efficiency.
References


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


