July 28, 2025
Tag:
1 Preface
Compound fertilizers are chemical fertilizers made by chemical methods and contain any two or three of the crop nutrients nitrogen, phosphorus and potassium.
Its function is to meet the comprehensive needs and balance of various nutrients required by agriculture under different production conditions. During the production process of compound fertilizers, manufacturers, considering cost issues, may use raw materials such as ammonium chloride and potassium chloride. Chlorine is a trace nutrient element. If the content of chloride ions is too high, it will affect seed germination and cause soil compaction. Therefore, the content of chloride ions in compound fertilizers is an important indicator for measuring the quality of compound fertilizers. This paper adopts the fully automatic potentiometric titration method to detect the chloride ion content in compound fertilizers. The operation is simple, the results are accurate and the precision is high.
2. Instruments and reagents
2.1 Instruments
JH-T7 fully automatic potentiometric titrator, silver composite electrode, 40-mesh sieve
2.2 Reagents
Silver nitrate standard solution (0.02mol/L), nitric acid solution (30%)
3 Experimental Methods
3.1 Experimental Procedures
Grind the fertilizer into fine powder with a mortar, pass it through a 40-mesh sieve, accurately weigh 2.5g of the fertilizer into a 250mL beaker, add 100mL of water, heat slowly to boiling, continue to boil gently for 10 minutes, cool to room temperature, transfer the solution to a 250mL volumetric flask, and dilute to the mark. Mix well, dry filter, and discard the initial part of the filtrate. Take 1mL of the filtrate into a titration cup, add 60mL of water and 2mL of nitrate solution, and titrate to the endpoint with 0.02mol/L silver nitrate titrant. At the same time, conduct a blank test.
3.2 Parameter Settings
| Titration mode | Dynamic titration |
stirring speed : |
5 |
|
Electrode equilibrium time: |
6s |
Pre-stirring time : |
6s |
|
Electrode equilibrium potentia: |
1mv |
Titration speed : |
标准 |
|
Minimum addition volume: |
0.04mL |
Pre-titration addition volume : |
6mL |
|
Final volume: |
10mL |
Pre-titration stirring time: |
30s |
| Potential spike |
150 |
Pre-controlled mv value: |
no |
4. Results and Discussion
4.1 Experimental Results
| Sample Name | Titrant Concentration (mol/L) | Sample volume (mL) | Blank Volume (mL) | Titration volume (mL) | Chloride ion (%) |
Average value (%) |
|
Compound fertilizer |
0.020 |
2.56009 |
0.360 |
9.320 |
62.04 |
62.27 |
|
9.368 |
62.40 |
|||||
|
9.368 |
62.36 |
4.2 Calculation Formula:
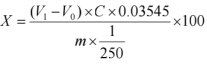
X- The content of chloride ions in the sample, expressed as a percentage (%);
V1- Volume of silver nitrate standard titration solution consumed in titrating the sample dilution solution, measured in milliliters (mL); V0- Volume of silver nitrate standard titration solution consumed blank, unit: milliliters (mL);
C - Concentration of silver nitrate standard titration solution, unit: moles per liter (mol/L);
4.3 Map
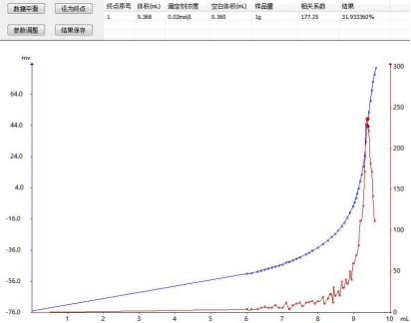
4.4 Conclusion
The chloride ion content in compound fertilizers was determined by fully automatic potentiometric titration. The absolute difference in parallel tests of the same sample was less than 0.4%, meeting the requirements of the national standard. The instrument can automatically control the titration process, determine the endpoint and process data, reducing the error caused by naked-eye determination of the endpoint. It features rapidity and simplicity. It can also reduce the contact between personnel and organic reagents, enhancing personnel safety.
References
[1] NY/T 1117-2010 Determination of Chlorine Content in Water-Soluble Fertilizers.[S]


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


