N55.6761September 12, 2025
Tag: Orphan drugs , Sales Resilience , Lifecycle Patterns
While orphan drugs are often recognized for their high pricing and regulatory incentives, their commercial value is frequently misunderstood. They might be viewed as short-term assets with limited market potential. However, a closer examination reveals a different narrative—one defined not by explosive growth, but by long-lasting, resilient revenue.
Unlike traditional mass-market drugs, orphan therapies target rare diseases with few or no therapeutic alternatives. Their uptake may be slower, but once adopted by specialized centers, they tend to entrench deeply into care protocols, enjoying prolonged clinical and commercial relevance. The market exclusivity granted under orphan drug legislation, combined with limited competitive entry, often allows these therapies to maintain sales levels over an extended period—beyond the typical lifecycle expectations seen in broader therapeutic areas.
Contrary to the notion that orphan drugs are limited in commercial potential, real-world sales data from 2025 reveal a compelling trend: orphan drugs not only sustain but often accelerate revenue long after their initial launch window. In an industry shaped by patent cliffs and payer pressure, the long-term sales resilience of these therapies is increasingly vital.
As illustrated in Figure 1, leading rare disease biopharmaceutical companies demonstrated robust and enduring commercial performance in Q1 2025.
Several key orphan therapies—such as Spinraza, Crysvita, and Voxzogo—continue to post substantial revenue growth many years after launch. Others like Trikafta and Vimizim maintain high baseline sales, underscoring their long-term clinical integration. In parallel, recently launched products (Elevidys, Skyclarys) are rapidly scaling, highlighting how orphan drug portfolios can layer mature assets with new growth engines. This pattern reflects a fundamental distinction from traditional therapeutics: a slower peak, longer plateau, and more resilient tail.
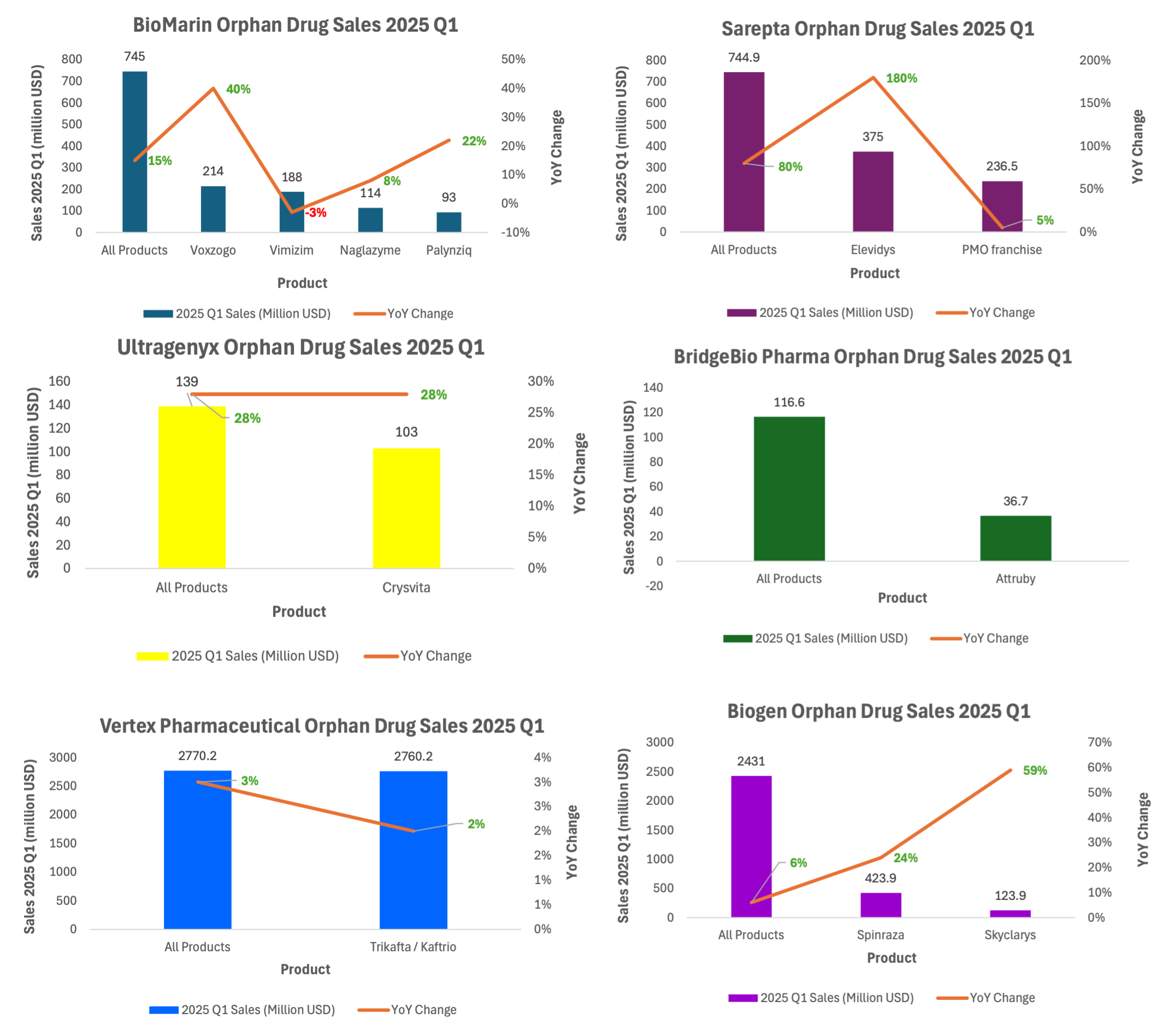
Figure 1. 2025 Q1 sales of leading orphan drug pharmaceuticals.
Among the clearest examples is BioMarin, whose rare disease portfolio has matured over two decades. In Q1 2025, Vimizim—approved in 2014 for Morquio A syndrome—still generated 188 million in revenue, despite a slight year-over-year decline. Naglazyme, approved in 2005 (20 years ago), posted $114 million quarterly revenue with a significant 8% YoY growth. Meanwhile, newer products like Voxzogo (approved in 2021) contributed over $214 million in the quarter, growing 40%. This layered revenue structure illustrates a key attribute of orphan drug portfolios: products mature slowly, overlap consistently, and compound into durable revenue.
The trend is echoed by Biogen whose orphan drug franchise contributes 23% quarterly revenue and may be arguably deemed as an orphan drug pharmaceuticals. Its orphan drug asset Spinraza—an antisense oligonucleotide approved in 2016 for spinal muscular atrophy (SMA)—reported $424 million in Q1 2025, up 24% year-over-year. This comes nearly a decade of post-launch, with new entrants like Skyclarys further expanding Biogen’s rare disease franchise.
Sarepta Therapeutics also exemplifies sustained growth with diversification. In Q1 2025, the company posted $745 million in total revenue—a staggering 80% increase from the prior year—driven by its newly approved gene therapy Elevidys for Duchenne muscular dystrophy (DMD), which accounted for $375 million. Its existing exon-skipping therapies (Exondys 51, Vyondys 53, Amondys 45) continued to contribute over $237 million.
Other companies showed similar dynamics:
Ultragenyx’s lead product Crysvita reached $103 million with 28% growth, despite launching in 2018.
Vertex saw $2.76 billion in Q1 revenue from Trikafta, approved in 2019, maintaining 2% YoY growth after many years of dominance in cystic fibrosis.
Chiesi, Ionis, and BridgeBio Pharma all maintained significant revenue contributions from their orphan portfolios, even where overall growth was tempered by prior-year licensing milestones or non-core factors.
While commercial attention often concentrates on launch velocity or peak-year sales, orphan drugs follow a different rhythm—one defined less by scale and more by consistency over time. Evidence from products approved over the past decade shows that orphan drugs frequently sustain or accelerate revenue well beyond the traditional launch window, often peaking later and declining more slowly.
Figure 2 displays Q1 2025 sales figures for selected FDA-approved orphan drugs that received marketing authorization between 2014 and 2024. The pale blue bars represent quarterly sales (in million USD), and the orange line tracks year-over-year (YoY) growth rates.
Only products with publicly disclosed sales data are included. Many approved orphan drugs—particularly those with very small patient populations, recent launches, or limited commercial reporting—are excluded due to the lack of available data.
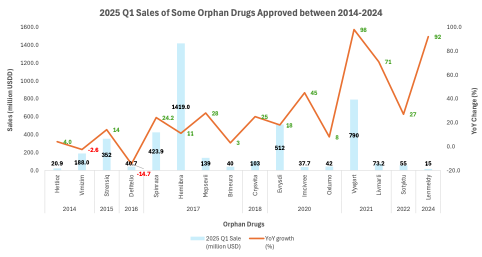
Figure 2. 2025 Q1 sales of orphan drugs (with disclosed revenue data) approved between 2014 and 2024.
Despite this limitation, the figure illustrates that several orphan drugs continue to deliver strong commercial performance well beyond their initial launch. For instance, Spinraza (approved in 2016) generated $424 million in Q1 2025, with a 24% year-over-year increase. Crysvita, launched in 2018, rose 25% in the same quarter. Even Strensiq (approved in 2015) continues to grow nearly a decade post-approval, posting a 14% YoY increase. Similar trajectories can be observed in Mepsevii (+28%) and Evrysdi (+18%), underscoring a clear pattern: many orphan therapies do not decline after early uptake but instead establish a long plateau of clinical use and economic contribution with two-digit growth rates.
This is not just a function of market exclusivity. These products are often irreplaceable in their therapeutic area, integrated into narrow specialist care networks, and insulated from generic pressure due to their biologic complexity or limited commercial viability for follow-on developers. Even among older products like Hetlioz and Strensiq, revenue maintains stable growth a decade after the launches.
Rather than seeking explosive growth, successful orphan drugs demonstrate long-tail durability—a form of commercial resilience built on clinical indispensability, not just regulatory protection.
1.Blockbuster Orphan Drugs in the 2024 Market
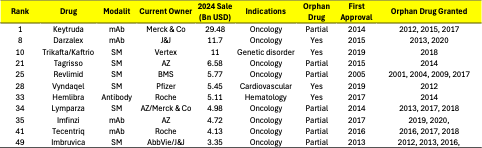
As orphan drugs continue to demonstrate commercial viability, a select group have crossed a threshold once thought unattainable for rare disease therapies—blockbuster status. In 2024, at least 11 of the world’s top 50 best-selling drugs were approved for orphan indications. Among them, four drugs—Darzalex, Trikafta, Vyndaqel, and Hemlibra—were developed exclusively for rare diseases. The rest, such as Keytruda, Revlimid, and Lymparza, include orphan indications among broader therapeutic uses.
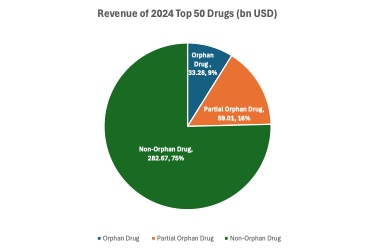
Figure 3. 2024 Revenues of Top 50 Drugs
Taken together, orphan drugs accounted for $92.29 billion in 2024 revenue among the top 50 products—representing 25% of total sales. Of this, $33.28 billion (9%) came from drugs with exclusively orphan indications, and another $59.01 billion (16%) from drugs with partial orphan drug.
These figures challenge the outdated notion that orphan drugs are niche assets with limited revenue potential. Trikafta’s$11 billion in annual sales—entirely from cystic fibrosis—has reshaped commercial expectations in rare disease drug development. Similarly, Darzalex and Hemlibra have demonstrated how therapies can scale globally while maintaining orphan exclusivity in major markets.
Even more significantly, hybrid-positioned therapies like Keytruda and Revlimid illustrate how orphan drug designation can contribute to multi-indication growth strategies. These drugs benefit from early regulatory incentives while expanding into broader patient populations, effectively extending both lifecycle and commercial durability.
As the pharmaceutical industry faces intensifying pressure to balance innovation, sustainability, and long-term value, orphan drugs offer an increasingly compelling strategic model. Far from being short-lived niche products, they have demonstrated a distinct lifecycle—marked by slower uptake, delayed peaks, and durable revenue tails. Their ability to sustain or even accelerate sales years after launch challenges conventional assumptions and highlights their role as stabilizing assets within biopharmaceutical portfolios.
Table 1. Exclusive Orphan Drugs Approved by FDA Between 2014 and 2024
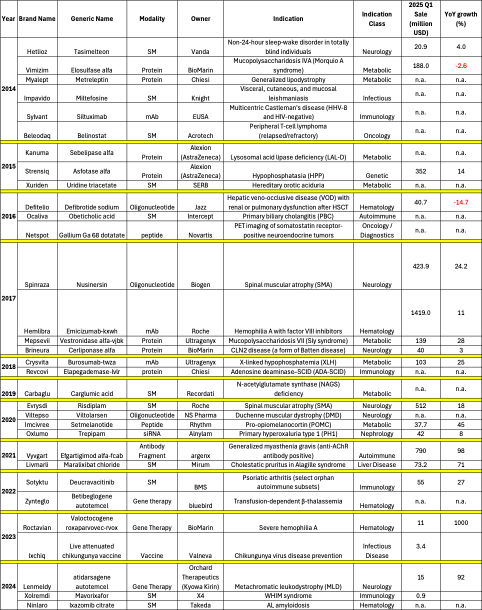
Table 2. Orphan Drugs from Top 50 Best-Selling Drugs of 2024


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


