N55.6761September 05, 2025
Tag: Peptide , GLP-1/GIP receptor agonists , Half-life extension
In the modern pharmaceutical landscape, few drug classes have witnessed a meteoric rise quite like peptide hormones. Leading the charge are semaglutide and tirzepatide, two incretin-based peptide drugs that have redefined the therapeutic and commercial potential of hormone-inspired medicines. Initially developed for type 2 diabetes, both agents have since expanded into the field of obesity, where their efficacy has unlocked one of the largest unmet medical needs of our time.
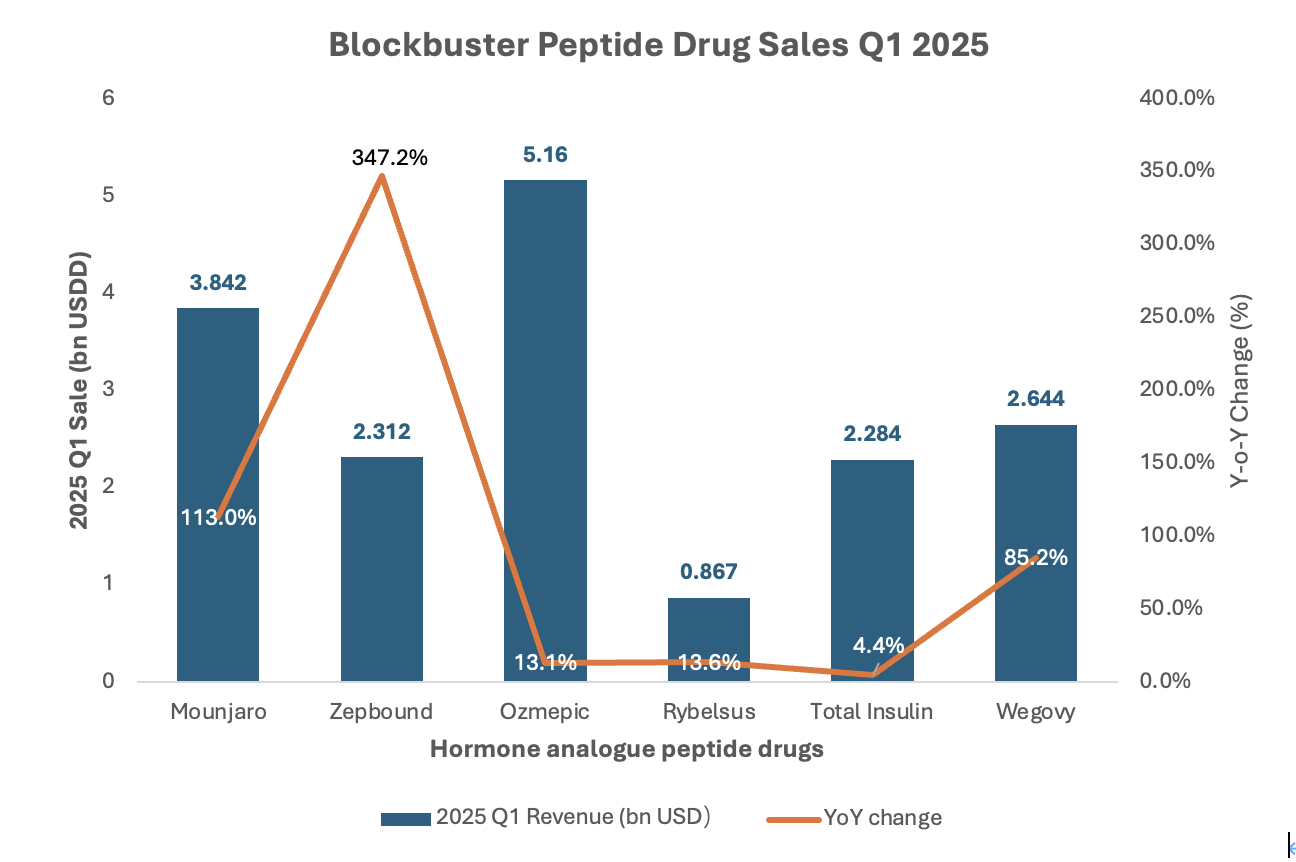
Figure 1. Super blockbuster peptide drug sales in Q1 2025.
As shown in Figure 1, sales of Ozempic (semaglutide) reached a staggering $5.16 billion, making it the top-selling peptidedrug worldwide. Its oral counterpart Rybelsus brought in another $867 million, while Wegovy, the weight-loss formulation of semaglutide, surged to $2.64 billion, marking an 85% year-over-year growth.
Even more remarkable is the rise of tirzepatide, a dual GIP/GLP-1 receptor agonist, with its two commercial brands—Mounjaro and Zepbound—generating $3.84 billion and $2.31 billion respectively. Zepbound alone grew by 347% compared to the same period last year, exemplifying the explosive demand for hormone-inspired peptide therapies in metabolic diseases.
These numbers not only reflect commercial triumphs but also signal a paradigm shift in how the pharmaceutical industry views endogenous peptide hormones. Once considered challenging to develop into drugs due to their short half-lives and poor oral bioavailability, these molecules are now being re-engineered into long-acting, highly selective therapeutics that dominate both diabetes and obesity markets.
This transformation underscores a powerful insight: the human hormonal system is one of the richest and most validated sources for drug discovery. By mining the structural, functional, and receptor-binding characteristics of endogenous peptides, scientists are unlocking new therapeutic opportunities across a range of indications—from metabolism to reproduction, and from neuroendocrine signaling to inflammation.
1.Hormone-Derived Peptides Anchor the Therapeutic Peptide Landscape
While peptides as a class are structurally and functionally diverse, hormone-derived peptides occupy a disproportionately influential position in the approved therapeutic space. Among the 122 peptide drugs approved by major regulatory agencies such as the FDA and EMA, 54 (44%) are derived from or inspired by endogenous hormones, as illustrated in Figure 2. These include analogues of glucagon, GLP-1, GIP, somatostatin, oxytocin, vasopressin, parathyroid hormone (PTH and PTHrP), α-MSH, and others, many of which were originally characterized as key regulators in human physiology.
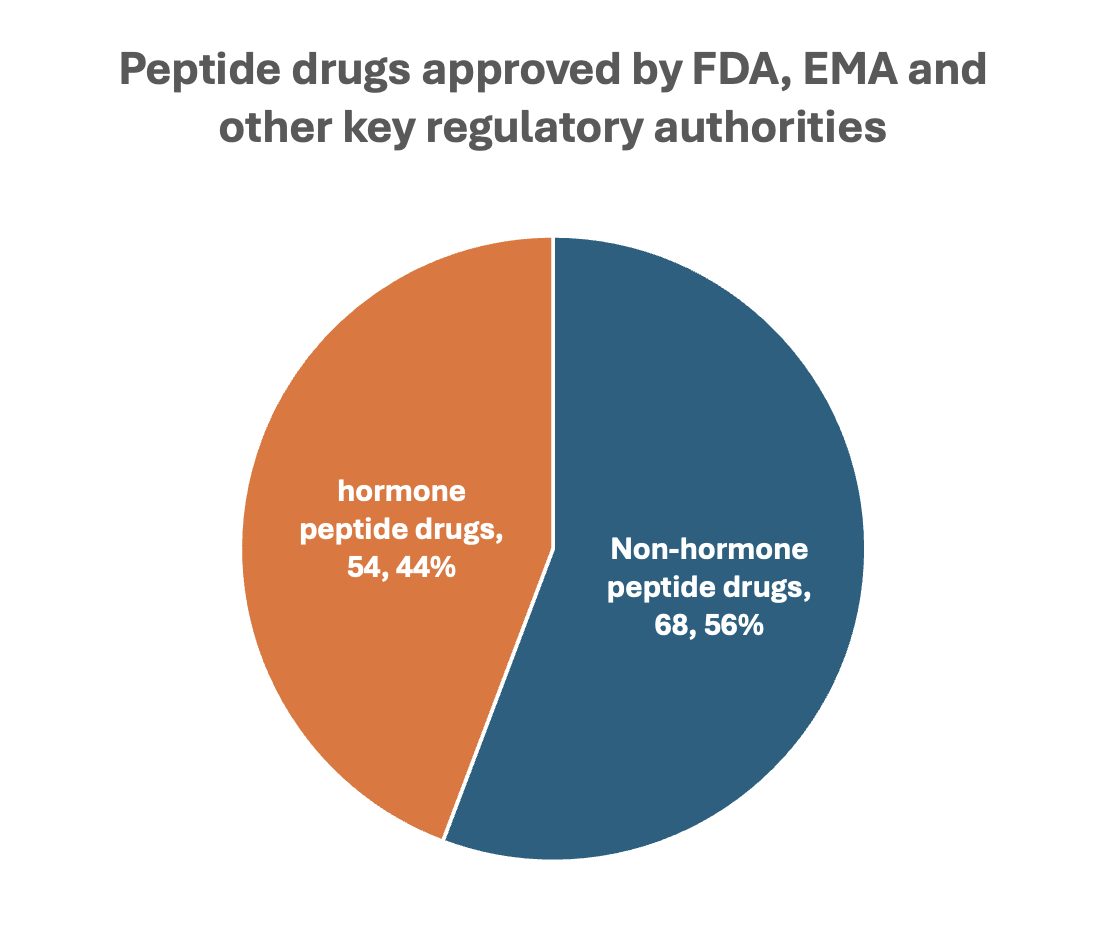
Figure 2. Hormone vs. non-hormone peptide drug approvals.
Despite constituting less than half of approved peptides numerically, hormone-based peptides account for the vast majority of commercial peptide drug revenue, led by GLP-1/GIP analogues in the diabetes and obesity space. But their relevance goes well beyond metabolism. In oncology and reproductive medicine, GnRH analogues like leuprolide and triptorelin have become long-standing standards of care. In cardiovascular and renal diseases, vasopressin analogues such as desmopressin and terlipressin remain clinical mainstays. In skeletal disorders, PTH and PTHrP analogues like teriparatide and abaloparatide are frontline therapies for osteoporosis.
The success of these therapies is rooted not only in their molecular ancestry, but also in the evolutionarily optimized signaling properties of peptide hormones: high target specificity, favorable safety profiles, and well-defined receptor pathways. Furthermore, many hormone receptors are already clinically validated, reducing development risk and enhancing translational efficiency.
Peptide hormones regulate nearly every axis of human physiology. Their pharmacological conversion into drugs preserves this system-level specificity. Based on the primary physiological role of their endogenous hormone origin, approved peptide drugs can be functionally grouped into six categories. This framework not only reflects therapeutic diversity but also illuminates the pharmacodynamic logic underlying hormone-based drug action.
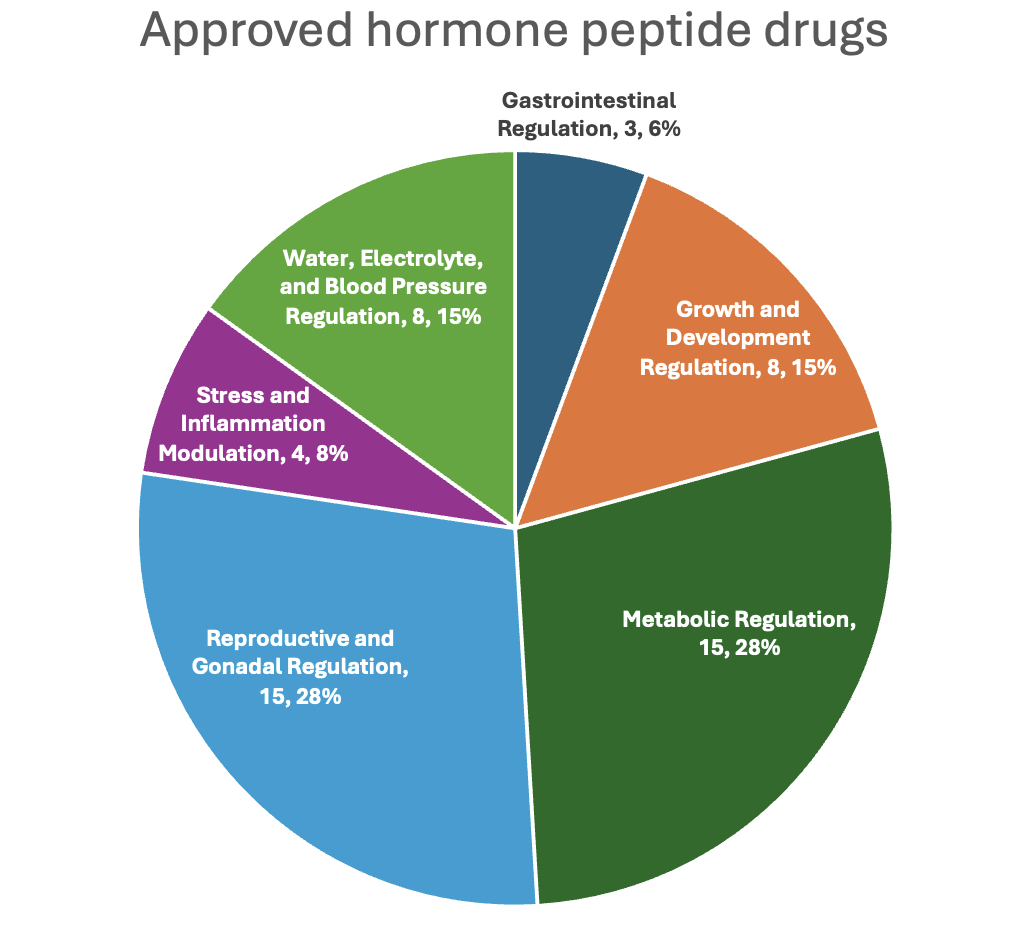
Figure 3. Functional classification of hormone-derived peptide drugs
As shown in Figure 3, reproductive/gonadal (28%) and metabolic (28%) regulation dominate the approved hormone peptide space, followed by water/electrolyte balance, growth regulation, and others.
·Representative hormones: GLP-1, GIP, glucagon, amylin, ghrelin, somatostatin
·Pharmacology: These peptides primarily act on GPCRs expressed in the pancreas, hypothalamus, and gastrointestinal tract to regulate insulin secretion, appetite, and nutrient absorption.
·Representative drugs:
Semaglutide and tirzepatide enhance insulin secretion, inhibit glucagon release, and slow gastric emptying. Tirzepatide targets both GLP-1 and GIP receptors.
Pramlintide mimics amylin to suppress post-meal glucagon and delay gastric transit.
Macimorelin activates ghrelin receptors and is used to assess adult growth hormone deficiency.
Somatostatin analogues such as octreotide, lanreotide, and pasireotide inhibit growth hormone and various gastrointestinal secretions, making them useful in conditions like acromegaly and neuroendocrine tumors.
·Representative hormones: GnRH, oxytocin
·Pharmacology: These peptides modulate the hypothalamic-pituitary-gonadal (HPG) axis or uterine smooth muscle. GnRH analogues regulate LH/FSH, while oxytocin analogues modulate myometrial contraction and milk ejection.
·Representative drugs:
Leuprolide, triptorelin, goserelin, and related GnRH analogues initially stimulate and subsequently suppress LH and FSH release and are widely used in hormone-sensitive cancers and reproductive disorders.
Degarelix acts as a GnRH receptor antagonist, leading to rapid suppression of testosterone without initial hormonal flare.
Oxytocin, carbetocin, and demoxytocin induce uterine contractions and are used for labor induction or postpartum hemorrhage management.
Atosiban functions as an oxytocin receptor antagonist and is used to delay preterm labor.
·Representative hormones: Vasopressin, natriuretic peptides, uroguanylin, angiotensin II
·Pharmacological functions: These peptides act primarily on renal tubules, vascular smooth muscle, and intestinal epithelium to control fluid retention, blood pressure, and electrolyte balance.
·Representative drugs:
Desmopressin, terlipressin, and ornipressin mimic vasopressin to promote water reabsorption or induce vasoconstriction in conditions like diabetes insipidus or hepatorenal syndrome.
Carperitide and nesiritide, analogues of ANP and BNP respectively, exert vasodilatory and natriuretic effects beneficial in acute heart failure.
Plecanatide, based on uroguanylin, stimulates intestinal fluid secretion through activation of guanylate cyclase-C receptors and is indicated for chronic idiopathic constipation.
Angiotensin II is used in vasodilatory shock to restore blood pressure through potent vasoconstriction and aldosterone stimulation.
·Representative hormones: Parathyroid hormone (PTH), parathyroid hormone-related protein (PTHrP), calcitonin, C-type natriuretic peptide (CNP), met-enkephalin
·Pharmacological functions: This group modulates skeletal growth, bone remodeling, and neuroendocrine growth pathways via osteoblast/osteoclast activity and chondrocyte proliferation.
·Representative drugs:
Teriparatide and abaloparatide intermittently activate PTH or PTHrP receptors to stimulate bone formation and are used in osteoporosis.
Calcitonin (including salmon and human forms) inhibits osteoclastic bone resorption and is used in conditions such as Paget’s disease and hypercalcemia.
Vosoritide, a CNP analogue, stimulates chondrocyte proliferation by activating natriuretic peptide receptor B (NPR-B) and is approved for achondroplasia.
Pralmorelin, a met-enkephalin analogue, is used diagnostically to evaluate growth hormone deficiency.
Tesamorelin, structurally related to GHRH, is approved for the treatment of HIV-associated lipodystrophy via growth hormone axis modulation.
·Representative hormones: ACTH, α-MSH
·Pharmacological functions: These peptides influence stress response, adrenal corticosteroid production, melanogenesis, and appetite regulation through the HPA axis and melanocortin receptors.
·Representative drugs:
Corticotropin and tetracosactide stimulate adrenal steroidogenesis via melanocortin 2 receptor (MC2R) activation, used diagnostically in adrenal insufficiency.
Afamelanotide activates MC1R in melanocytes to increase melanin production and prevent phototoxicity in erythropoietic protoporphyria.
Setmelanotide acts on the MC4R pathway in the hypothalamus to reduce hunger and is approved for rare genetic obesity syndromes.
·Representative hormones: Secretin, gastrin-like peptides
·Pharmacological functions: These peptides regulate gastric acid secretion, pancreatic enzyme release, and intestinal motility, primarily via GPCRs on epithelial and endocrine cells.
·Representative drugs:
Secretin (porcine and recombinant human) is used in diagnostic testing of pancreatic exocrine function and gastrinoma.
Pentagastrin stimulates gastric acid secretion and is also used diagnostically in endocrine disorders.
Endogenous peptide hormones are highly potent and selective molecules evolved for precise physiological regulation. However, their direct use as drugs is often limited by poor pharmacokinetics, susceptibility to enzymatic degradation, and rapid renal clearance. To overcome these barriers, pharmaceutical innovation has developed a range of structural and formulation strategies to transform fragile peptide messengers into clinically viable therapeutics.
Below are the most widely used strategies for optimizing hormone-derived peptide drugs:
·Half-Life Extension through Lipidation or PEGylation
Native peptide hormones are usually short-lived, with half-lives ranging from minutes to hours. Conjugating the peptide to a fatty acid chain or polyethylene glycol (PEG) moiety can significantly extend plasma exposure by enhancing albumin binding or reducing renal filtration.
Semaglutide incorporates a C18 fatty diacid moiety via a hydrophilic linker, conferring a half-life of ~7 days, allowing once-weekly dosing. Tirzepatide incorporates a C20 fatty diacid moiety linked via a hydrophilic spacer to a modified peptide backbone, which enhances albumin binding and prolongs its plasma half-life to approximately 5.4 days.
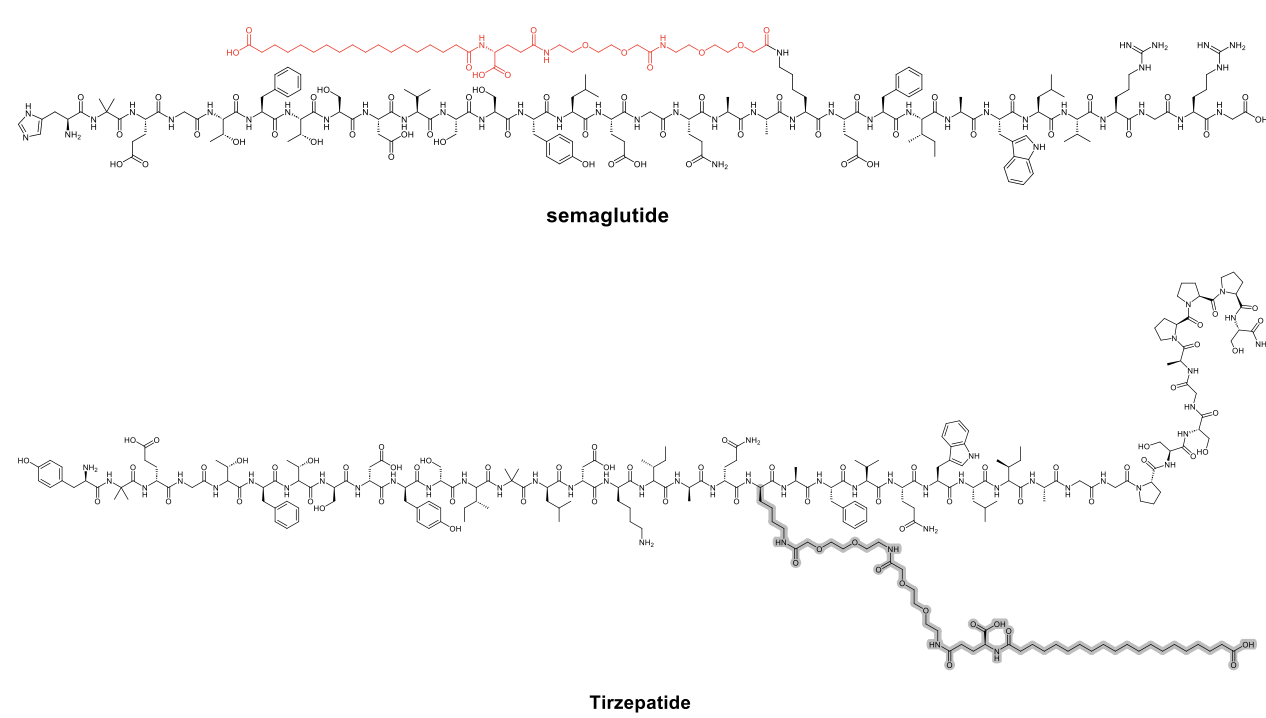
Several other analogues, such as efpeglenatide or pegcetacoplan, rely on PEG-based or Fc-fusion mechanisms.
·Backbone Cyclization and Non-Natural Amino Acid Substitution
Cyclization—either head-to-tail or via disulfide bridges—can stabilize secondary structures, protect against exopeptidases, and enhance receptor affinity. Incorporation of D-amino acids or other non-natural residues can further resist enzymatic cleavage.
·Receptor Selectivity Engineering
While native hormones often act on multiple receptor subtypes, engineered analogues can achieve greater selectivity, thereby reducing side effects and expanding therapeutic windows.
Tirzepatide is designed to co-activate GIP and GLP-1 receptors in a biased manner, enhancing weight loss efficacy beyond traditional GLP-1 agonists. Setmelanotide selectively targets MC4R, avoiding MC1R and MC5R-associated toxicities observed with other melanocortin analogues.
·Oral or Non-Invasive Delivery Technologies
Peptides are traditionally injected due to poor gastrointestinal absorption. However, novel technologies, including co-formulation with absorption enhancers, microneedle patches, and depot systems, are extending delivery options.
Rybelsus (oral semaglutide) employs SNAC, a sodium salt-based absorption enhancer, to enable gastric absorption of the peptide.
·Diagnostic Adaptation of Hormone Receptor Ligands
Some peptides are adapted for imaging or diagnostic testing by radiolabeling or structure truncation, preserving receptor binding while eliminating full agonist activity.
Indium In-111 Pentetreotide and Dotatate Ga-68 are radiolabeled somatostatin analogues for neuroendocrine tumor imaging. Macimorelin, a ghrelin receptor agonist, stimulates growth hormone release from the pituitary and is used to diagnose adult growth hormone deficiency.
Taken together, these strategies illustrate that successful hormone-derived peptide drug development is not a mere extension of biology, but a deliberate engineering effort. By leveraging structure–function relationships, medicinal chemists have turned ephemeral signaling molecules into long-acting, tissue-targeted, and highly controllable therapies.
The extraordinary commercial and clinical success of peptide hormones such as GLP-1, GIP, and amylin analogues has reshaped the pharmaceutical landscape. Yet, these represent only a fraction of the endogenous hormonal peptidome. A growing body of research continues to uncover promising peptide candidates from underexplored endocrine, neuroendocrine, and paracrine systems. The next generation of hormone-inspired peptide drugs will likely come not just from optimizing known pathways, but from unlocking new hormonal circuits with therapeutic relevance
Table 1. Hormone-based phase 3 peptide assets
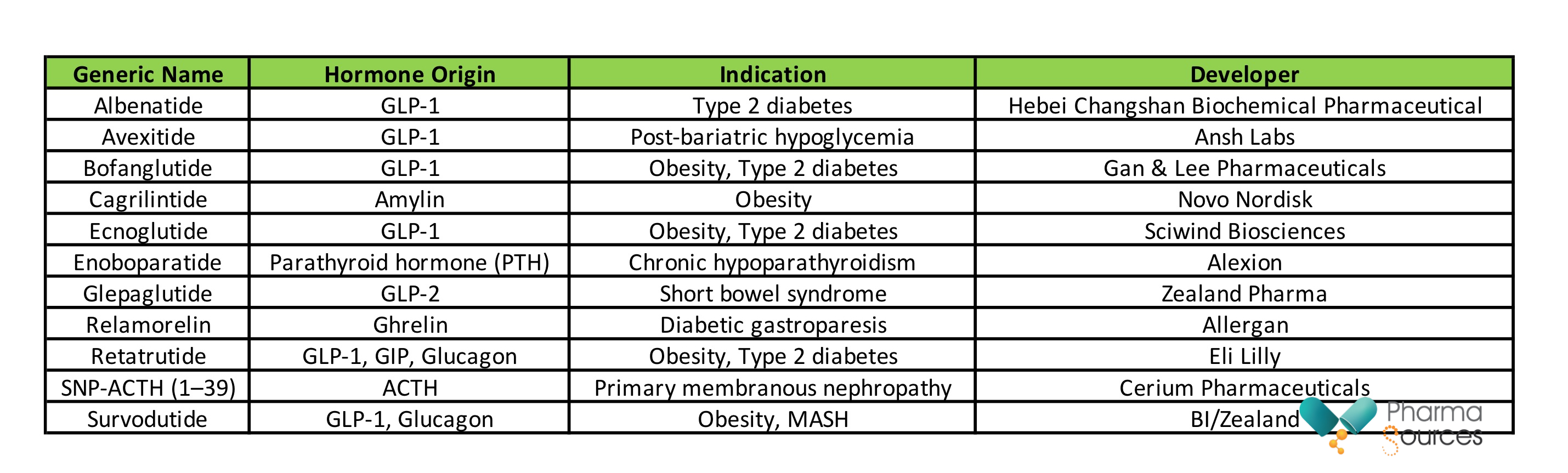
A review of peptide assets currently in Phase 3 clinical development reveals a strong dominance of hormone-derived candidates, reflecting both biological validity and druggability. As summarized in Table 1, GLP-1-based analogues remain the most crowded field, with candidates such as albenatide, avexitide, ecnoglutide, and bofanglutide advancing toward potential regulatory approval. These agents are differentiated by modifications in half-life extension, co-agonist architecture, or indication specificity.
Notably, newer hormone axes are also being harnessed:
Cagrilintide represents an analogue of amylin, targeting the amylin receptor to reduce appetite and body weight.
Relamorelin, a ghrelin receptor agonist, is being developed for diabetic gastroparesis.
Enoboparatide builds on parathyroid hormone biology, advancing treatment options for hypoparathyroidism.
SNP-ACTH (1–39) revives adrenocorticotropic hormone (ACTH) pharmacology for autoimmune renal diseases.
Survodutide expands the GLP-1/glucagon co-agonist concept into the realm of liver diseases, including metabolic dysfunction-associated steatohepatitis (MASH).
These examples highlight a few core themes in future hormone peptide drug development:
Multi-receptor engagement: Drugs like retatrutide and survodutide simultaneously target GLP-1, GIP, and glucagon receptors, aiming for synergistic metabolic outcomes such as weight loss and hepatic fat reduction.
Indication expansion beyond classical endocrinology: Hormone-inspired peptides are now being developed not only for diabetes and osteoporosis, but also for gastrointestinal disorders, neurodegeneration, rare autoimmune diseases, and liver fibrosis.
Improved formulations for tolerability and compliance: Once-weekly or monthly injectables, as well as efforts in oral or transdermal delivery, are transforming the usability of peptide therapies.
Looking forward, several underutilized hormones may form the next wave of innovation. These include:
Neuropeptide Y (NPY) and PYY, both involved in appetite and energy balance;
Kisspeptin, a gatekeeper of reproductive hormone release;
Relaxin, with emerging evidence in cardiac and fibrotic diseases;
Motilin and neurotensin, with roles in GI motility and neuromodulation;
and Substance P or CRH, which could be leveraged in neuroinflammation and pain.
As peptide synthesis, stabilization, and delivery technologies continue to evolve, and with artificial intelligence accelerating peptide optimization, the human hormonal system remains one of the most clinically validated and underexploited sources for novel therapeutics. The success of semaglutide and tirzepatide is not the end of the hormone peptide story—but merely its beginning.
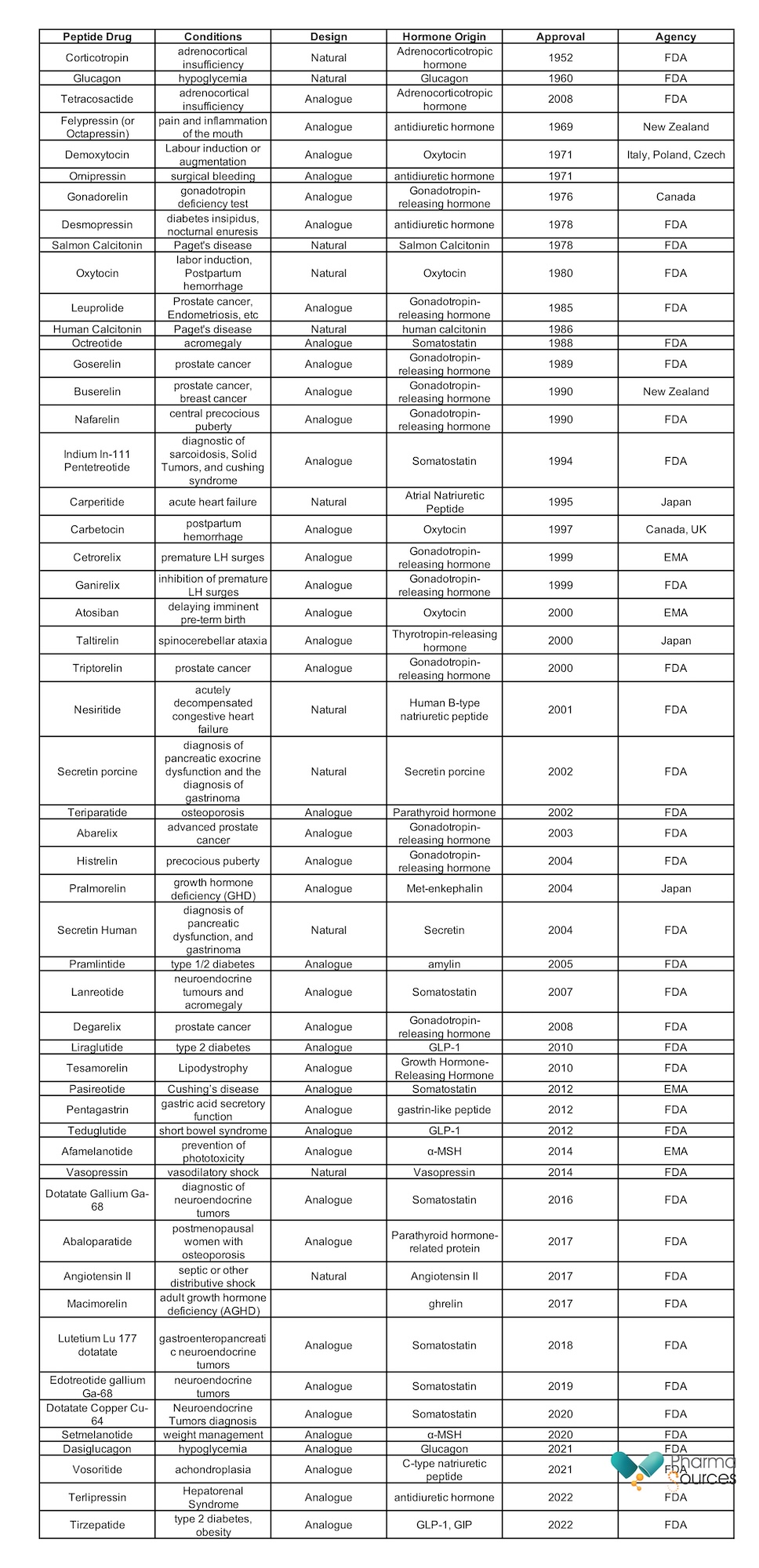
Appendix: Marketed hormone-based peptide drugs


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


