N55.6761June 19, 2025
Tag: United States , Drug Origin , API , Generics
Recently, the United States Pharmacopeia (USP) published a report that brings to light a concerning reality: approximately 85% of active pharmaceutical ingredients (APIs) used in brand-name prescription drugs in the United States are imported. The timing of this revelation is particularly notable, as it coincides with the Trump administration's proposed tariff policy on pharmaceuticals - an alert to the global pharmaceutical industry.
According to the report, only 15% of APIs used in branded drugs of the United States are manufactured domestically. The majority come from abroad, with Europe accounting for 43%, India 2%, and China 3% (Figure 1). This heavy reliance on foreign sources suggests that the proposed tariff measures could bring significant challenges to pharmaceutical companies, including soaring costs and constrained supply chains.
Under the Trade Agreements Act, the country of origin for pharmaceuticals is typically determined by the source of APIs, unless the product undergoes "substantial transformation" in another country. As a result, even if the finished dose form is manufactured and packaged in the United States, it may still be classified as a "foreign product" subject to tariffs if its APIs are sourced from abroad.
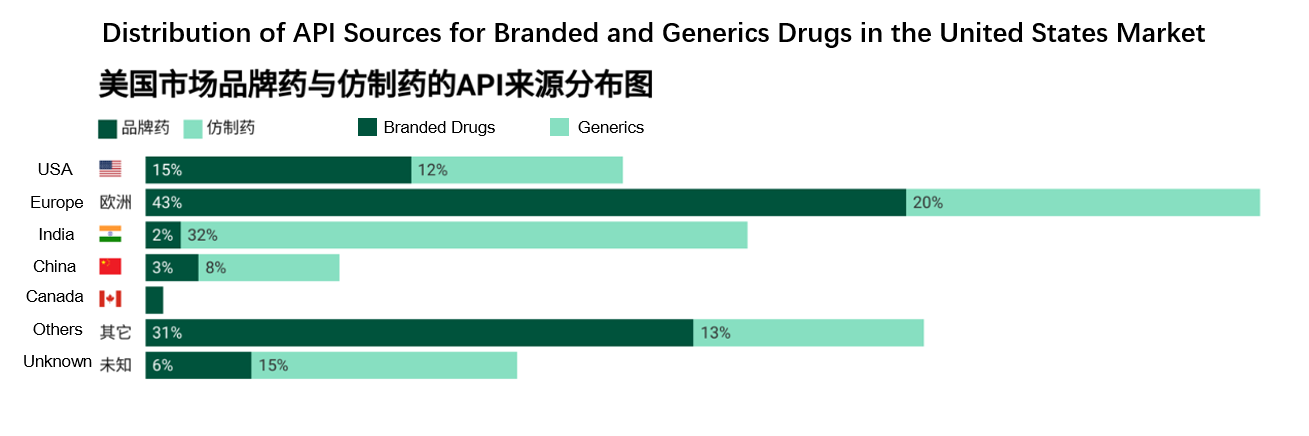
Figure 1. Composition of API Sources for Branded Drugs and Generics in the United States Market. (Source: United States Pharmacopeia)
Europe has long held a prominent position in global API production, renowned for its high quality standards and technological sophistication. It remains a key player, particularly in the fields of high-end small molecule APIs and complex chemical synthesis. Countries such as Germany, Switzerland, and Italy host world-class GMP manufacturing bases, and have been long-standing suppliers of critical raw materials to major branded pharmaceutical companies of the United States.
According to data from the USP report, 43% of the APIs used in branded drugs of the United States are sourced from Europe. In contrast, India and China contribute just 2% and 3%, respectively, to the API supply for branded drugs. This heavy reliance on European sources makes API enterprises in Europe the most vulnerable if tariff policies are implemented. As Marta Wosińska, a prominent health economist and former FDA official, bluntly stated, "Europe has far more API manufacturing plants than the United States - if tariffs are imposed, they will clearly be hit the hardest."
While the API supply for branded drugs is largely dominated by the United States and Europe, the generics market tells a different story - its API supply is heavily reliant on India and China (Figure 1).
· India accounts for 35% of United States generics API imports, leveraging a significant cost advantage in the off-patent drug field.
· China accounts for 8%. Although its share is relatively small, it holds a dominant position globally in the field of upstream raw materials such as starting chemicals.
· Europe also contributes 18%, primarily supplying certain complex APIs and high-end generics.
Compared with the highly profitable branded drugs field, pharmaceutical companies of generics already operate on very thin margins. A significant rise in API costs could render many of them commercially unsustainable. At the same time, the United States lacks sufficient domestic production capacity for generic API. As a result, even if there is a push toward "de-globalization", there are no viable short-term alternatives. This could lead to increased market volatility in the medium to long term, and potentially trigger drug shortages for certain products.
API tariffs affect not only the cost structure of pharmaceuticals, but also place pressure on the internal pricing and tax planning of transnational pharmaceutical companies.
In international business, the transfer of APIs between different subsidiaries within a group requires transfer pricing, with prices set according to the "arm's length principle". Once APIs are subject to tariffs, enterprises may attempt to artificially lower internal settlement prices to reduce tax burdens. However, this may violate existing tax regulations and attract scrutiny from tax authorities, increasing compliance risks. Marta Wosińska pointed out: "If APIs can only be imported, while finished drugs are completed in the United States, enterprises will face significant pressure on price adjustments."
Although China holds a relatively small share in APIs used in branded drugs, it remains one of the world's largest suppliers of intermediates and starting chemicals field. At the very upstream stage of API synthesis, many global CDMO enterprises are deeply reliant on Chinese manufacturing.
This also means that the Trump administration's tariff investigation may extend further upstream to cover starting chemicals and the stage of chemical intermediates. If Chinese products in these categories are included in the tariff list, it could cause structural disruption to the global upstream pharmaceutical supply chain. On the other hand, such policy shifts may also push Chinese CDMO enterprises to enhance their global compliance capabilities, accelerate overseas manufacturing expansion, and diversify their client base - strengthening their international position in both low- to mid-end and certain high-end APIs.
The tariff storm originating in the United States is prompting pharmaceutical companies worldwide to rethink their supply chain strategies. Several key trends are emerging:
· "Localized manufacturing" is back on the agenda, with the United States likely to introduce incentives to bring API back home.
· "Nearshore outsourcing" is gaining traction, with countries like Mexico, Canada, and those in Eastern Europe emerging as potential API production capacity site;
· The "dual-center + multi-node" model is gaining popularity, with major pharmaceutical companies tending to establish dual centers (United States/Europe + Asia) and multiple nodes (low-risk + low-cost) in their supply networks;
· Compliance pressure across the supply chain is increasing, and enterprises need to strike a more precise balance among taxation, environmental protection, and regulatory requirements.
The United States Pharmacopeia (USP) also conducted an analysis of the global origins of finished dose forms (FDFs) used in prescription drugs of United States, leading to several key findings (Figure 2):
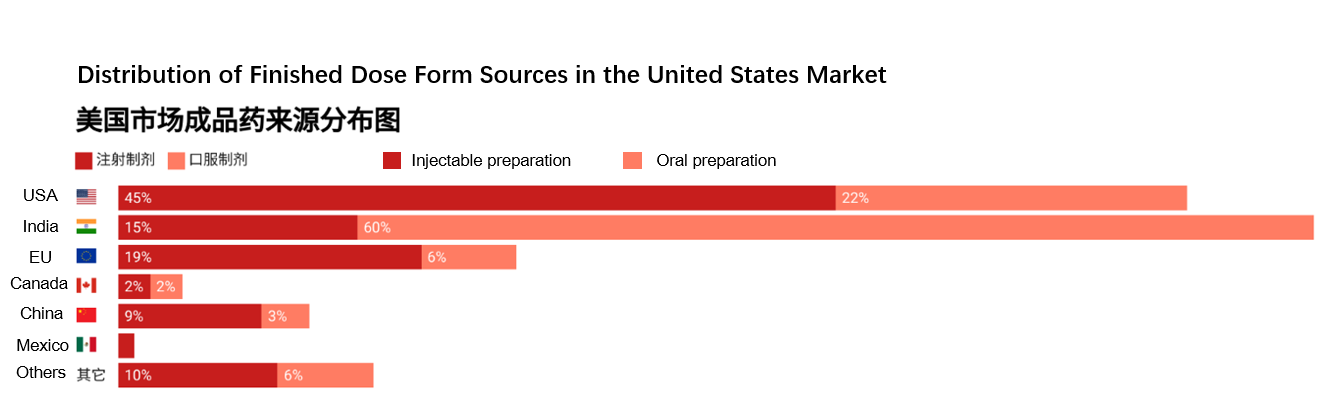
Figure 2. Composition of Finished Dose Form Sources in the United States Market (Source: United States Pharmacopeia)
· India and the United States are the primary producers of FDFs in the United States market. Injectable and oral preparations from India account for 15% and 60% of the market respectively.
· For injection dosage, United States-made products represent the largest share, comprising 45% of the total.
· Injectable preparation and oral preparation made in China account for 9% and 3% of their respective markets in the United States.
· Overall, market shares have remained relatively stable over the past two years, with no significant fluctuations.
The USP notes that the high geographic concentration of pharmaceutical manufacturing capacity - regardless of whether it occurs abroad or within the United States - increases the risk of supply disruptions. Enhancing the geographic diversity of the US drug manufacturing bases is therefore seen as a critical strategy to reduce the risk of future drug shortage.
In 2025, the global pharmaceutical export landscape is expected to remain highly concentrated, with a handful of countries dominating the international market. According to data released by World Population Review, Germany, Switzerland, and the United States rank as the top three pharmaceutical exporters worldwide, each demonstrating distinct advantages in industrial capacity, innovation, and high-value-added drug exports. Other European nations such as Belgium, Ireland, and the Netherlands also secure positions within the top ten, thanks to their robust infrastructure, supportive policies, and global layout. This ranking not only reflects the current structure of the global pharmaceutical supply but also underscores the critical importance of supply chain stability in ensuring resilient global health systems.
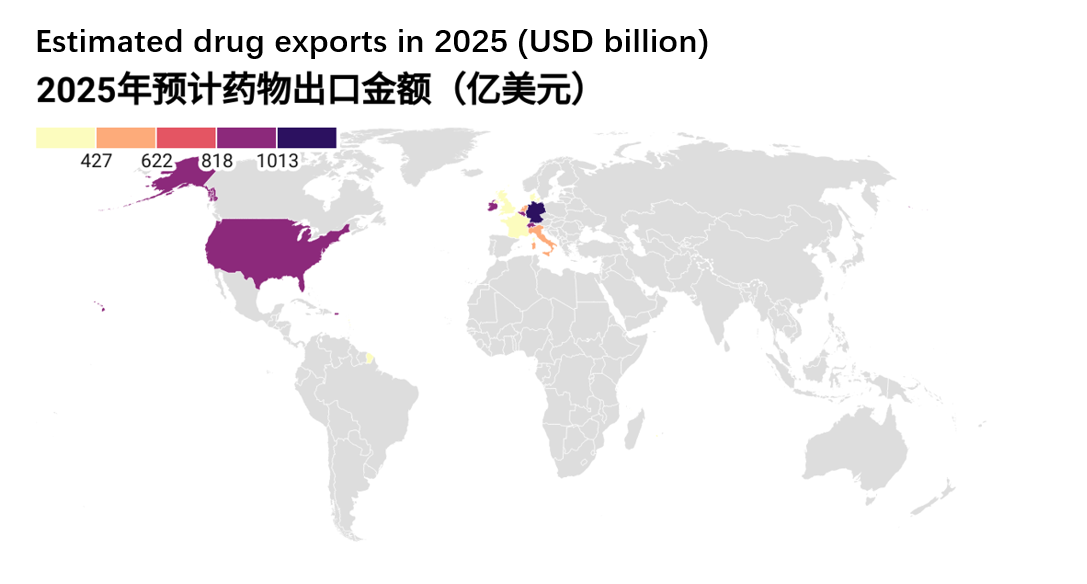

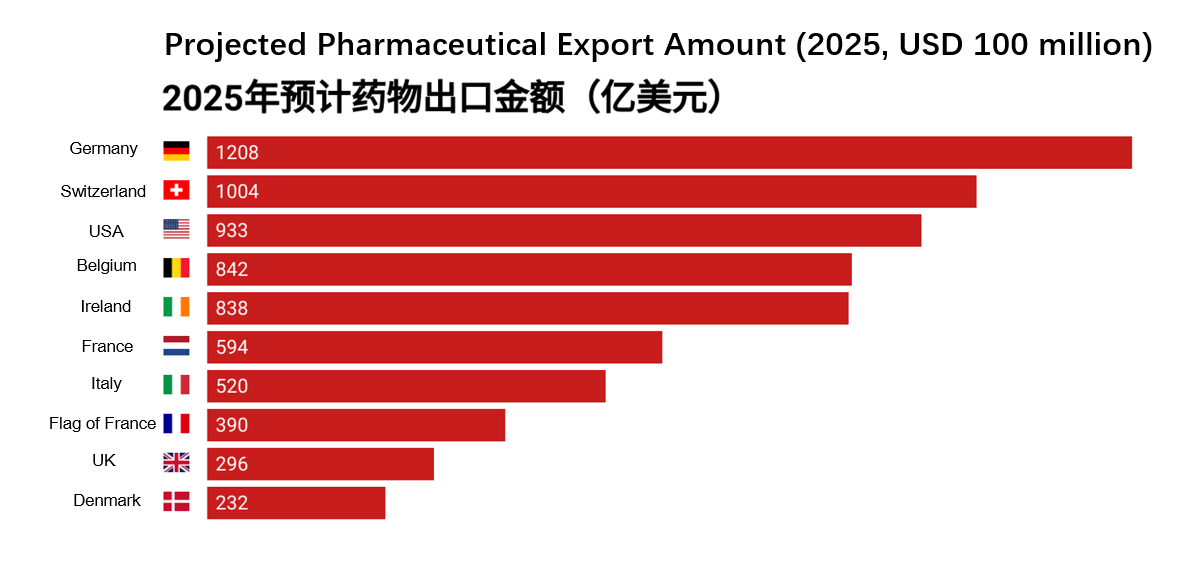
Figure 3. Projected Global Pharmaceutical Export Rankings (2025) (Source: Urge Money)
As the backbone of European manufacturing, Germany leads global drug exports, leveraging its strong industrial base and advanced pharmaceutical system. Its dominant position is reinforced by powerful brand recognition, stringent quality standards, and its central EU location.
Switzerland, though small in size, is a veritable pharmaceutical powerhouse. With pharmaceutical giants like Novartis and Roche headquartered there, the country specializes in high-value innovative drugs. Its stable economy and robust intellectual property framework further attract substantial capital investment.
The United States, serving as the global engine for biotechnology and pharmaceutical innovation, continues to see rising export volumes. Despite its vast domestic market, high-tech therapeutics and vaccines have emerged as key growth drivers in exports.
Belgium serves as the logistical hub of Europe's pharmaceutical industry, boasting world-class infrastructure and vaccine manufacturing capabilities. It plays an indispensable role in the global pharmaceutical supply chain.
Ireland has emerged as a global pharmaceutical companies manufacturing hub, benefiting from lower enterprise tax rates and a highly skilled workforce. Industry leaders including Pfizer, Eli Lilly, and Johnson & Johnson have established production facilities here, generating remarkable export volumes.
The Netherlands has built a highly efficient pharmaceutical sector, leveraging the Port of Rotterdam and world-class logistics networks to establish strong competitiveness in generics and conventional drug production. Notably, the European Medicines Agency (EMA) is headquartered in Amsterdam.
Italy combines traditional manufacturing strengths with cutting-edge biotech innovation, achieving rapid vaccine production expansion in recent years. This has significantly elevated its role in the global health emergency response system.
France, home to global pharmaceutical giants like Sanofi, has consistently ranked among the world's top drug-producing nations. Its export competitiveness stems from three key pillars: R&D capabilities, government support, and cooperation within the EU.
The United Kingdom has maintained its leadership in innovative drugs and biotechnology even post-Brexit, with world-class research institutions like the University of Oxford continuing to drive its vitality in global pharmaceutical exports.
Denmark secures its top-ten position primarily through Novo Nordisk's global dominance in diabetes and obesity treatments field with its Semaglutide product series. The country's advanced manufacturing capabilities and sustained innovation further propel its export growth.
Ref.
Over half of the active pharmaceutical ingredients (API) for prescription medicines in the U.S. come from India and the European Union. USP. 17. 04. 2025.
India and the United States manufacture most finished medicines for the U.S. market. USP. 19. 02. 2025.
20. 04. 2025. Money, U. Top Pharmaceutical Exporting Countries in 2025. https://urgemoney.com/top-pharmaceutical-exporting-countries-in-2025/


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


