David Orchard-WebbJune 23, 2025
Tag: Pharmaceutical Market Trends , Top Selling Drugs 2025 , Drug Sales Forecast , Keytruda , Ozempic , Dupixent , Skyrizi , Mounjar
In the rapidly changing pharmaceutical environment, identifying which therapies are positioned to dominate the market is critical for investors, marketers, healthcare providers, and legislators. As chronic illness rates rise and access to specialist care expands in both developed and emerging nations, blockbuster medications continue to redefine industry norms. According to current market research, five drugs are expected to dominate worldwide sales in 2025: Keytruda, Ozempic, Mounjaro, Dupixent, and Skyrizi (Mikulic, 2025). Each of these drugs offers a substantial therapeutic development in areas with high unmet medical need, such as cancer, diabetes, and immunology.
In this update, we look at the conditions that have contributed to the sales success of these five drugs, as well as the larger implications for the pharmaceutical business.
Merck & Co.'s Keytruda has changed the oncology industry since its approval as an immune checkpoint inhibitor that targets PD-1. The medication is expected to be the world's best-selling pharmaceutical in 2025, with predicted annual sales topping $30 billion (Mikulic, 2025).
Keytruda's success is primarily due to its growing array of indications. It was first licensed for melanoma and non-small cell lung cancer (NSCLC), however it is currently used to treat over 20 other cancer types (Merck, 2015). Its potential to improve survival rates in difficult-to-treat malignancies, either as monotherapy or in conjunction with chemotherapy, has resulted in widespread use in both early- and late-stage diseases. Furthermore, Keytruda's use in adjuvant and neoadjuvant cancer therapy protocols has increased its commercial reach.
Approvals for early-stage malignancies, including as renal, head and neck, and triple-negative breast cancer, are projected to drive future growth. Merck's robust clinical development program continues to investigate nearly 1,600 studies, guaranteeing the drug's relevance and market leadership for many years to come.
Ozempic, Novo Nordisk's GLP-1 receptor agonist for type 2 diabetes, is expected to earn more than $22 billion in global sales during 2025 (Mikulic, 2025). The drug's outstanding commercial performance shows the increased need for medicines that address both hyperglycemia and weight reduction; two critical drivers in diabetes management.
While Ozempic was initially released as a weekly injection for glycemic management, its side effect of aiding weight loss has fuelled off-label prescription and media interest (Wojtara, 2023). This dual action places the medicine at the confluence of two important public health issues; diabetes and obesity.
Novo Nordisk's targeted marketing, along with favorable cardiovascular outcomes data, has expanded marketing permission to encompass comorbid illnesses such as serious heart problems specifically in adults with obesity or overweight (FDA, 2024). As obesity medication achieves regulatory and payer acceptability worldwide, Ozempic's sales momentum is projected to accelerate.
Eli Lilly's Mounjaro, the newest addition to the top five, is expected to generate more than $19 billion in sales during 2025 (Mikulic, 2025). Mounjaro, approved in 2022, is a first-in-class dual GIP/GLP-1 receptor agonist for type 2 diabetes (Lilly, 2022).
Mounjaro has distinguished itself with better effectiveness results, displaying remarkable A1c reductions and weight loss when compared to other GLP-1 medicines. Its market trajectory mimics, and in some cases outperforms, Ozempic's, notably in weight control. Patients in clinical studies lost an average of more than 20% of their body weight, establishing the medicine as a game-changer in the growing obesity treatment sector (Lilly, 2022).
Lilly's ambitious growth plan, which includes numerous Phase 3 studies addressing obesity, cardiovascular risk, and metabolic disorders, places Mounjaro as a potential category leader. Regulatory approval for obesity indications under the brand name Zepbound have sparked more investor and physician interest (FDA, 2024b).
Dupixent, an interleukin-4 and interleukin-13 pathway inhibitor developed by Sanofi and Regeneron, is expected to generate over $16 billion in sales during 2025 (Mikulic, 2025). Dupixent, which was first approved for moderate-to-severe atopic dermatitis, has also been FDA approved to treat a further seven indications; asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis, pruigo nodularis, COPD, chronic spontaneous urticaria (CSU), and bullous pemphigoid (Drugs, 2025) (Regeneron, 2025) (Figure 1).
Dupixent's quick acceptance is due to its outstanding effectiveness, safety, and durability evidence across a wide range of inflammatory illnesses, many of which previously had few biologic choices (Drugs, 2025). Its broad therapeutic profile, steroid-sparing potential, and pediatric indications have resulted in widespread prescriber acceptance.
Sanofi and Regeneron have made significant investments in extending the drug's authorized applications, with late-stage trials underway for conditions with type 2 inflammation, including chronic pruritus of unknown origin and lichen simplex chronicus (Regeneron, 2025). Dupixent demonstrates the commercial viability of multi-indication immunotherapies in chronic illness markets.
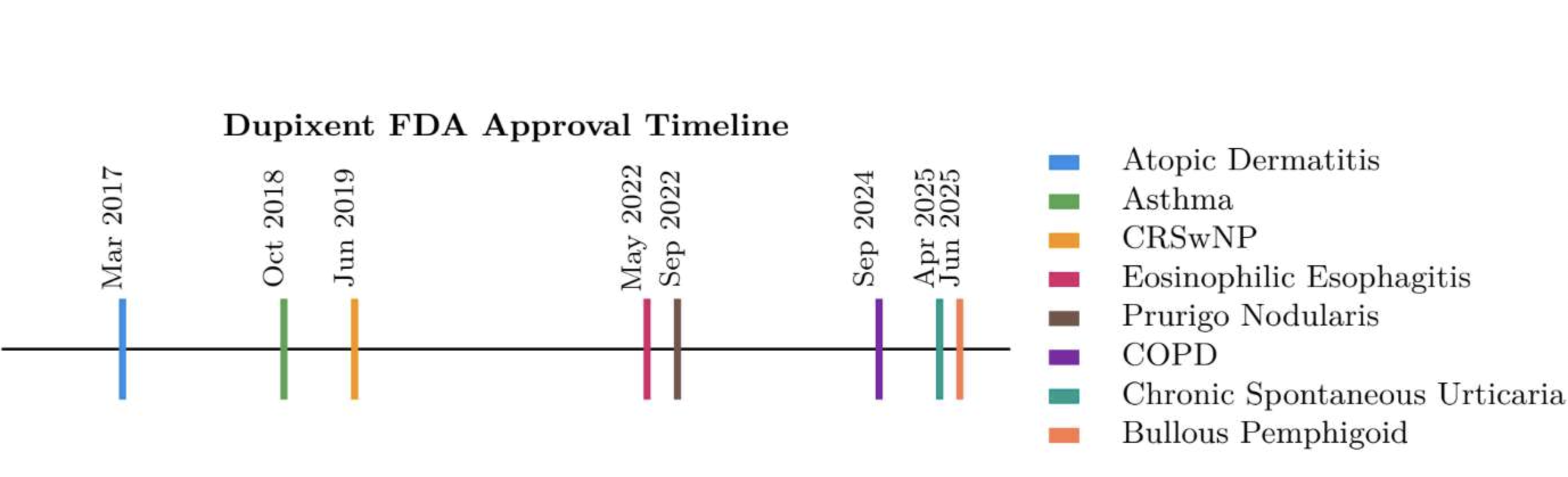
Figure 1: Dupixent FDA regulatory approvals by year. CRSwNP; Chronic Rhinosinusitis with Nasal Polyposis, CSU; Chronic Spontaneous Urticaria.
AbbVie's Skyrizi, an interleukin-23 inhibitor for certain chronic immune-mediated diseases of the epithelium, rounds out the top five, with worldwide sales expected to approach $14 billion during 2025 (Mikulic, 2025). The drug's remarkable success highlights the continued rise of immunology, particularly in the biologics area.
Skyrizi, which was launched in 2019 for plaque psoriasis, quickly gained market share thanks to its high skin clearance rates, simple quarterly maintenance dose, and good safety profile when compared to earlier TNF inhibitors (AbbVie, 2019). Three further approvals in active psoriatic arthritis, Crohn's disease, and ulcerative colitis have provided new cash sources (AbbVie, 2024).
AbbVie intentionally positioned Skyrizi alongside Rinvoq (upadacitinib) to compensate for post-Humira biosimilar losses (Liu, 2021). The drug's effectiveness has confirmed the IL-23 pathway as a critical target in immunoinflammatory illnesses, with further promise in other autoimmune ailments such as hidradenitis suppurativa (Kimball, 2023).
These five medications dominate the worldwide pharmaceutical industry, reflecting various major trends. First, there is a clear movement toward specialty biologics that treat chronic, complicated illnesses with significant unmet needs. Oncology, diabetes, and immunology continue to receive the majority of pharmaceutical R&D funding, yielding breakthrough treatments with multibillion-dollar sales potential.
Second, combination indications and label extensions have developed as highly effective growth methods. Drugs such as Keytruda and Dupixent demonstrate this strategy, with continuous clinical development programs expanding their market reach beyond initial approval.
Finally, the incorporation of patient-centric outcomes, such as weight reduction, better quality of life, and decreased steroid reliance, has become a critical component in product uptake and pricing discussions in developed countries.
The pharmaceutical industry in 2025 will be defined by a small number of high-performing biologics and specialized medicines that address the world's most pressing health issues. Keytruda, Ozempic, Mounjaro, Dupixent, and Skyriziall demonstrate the financial benefits of clinical innovation, strategic indication extension, and responsive market access initiatives.
These developments highlight the need of investing in immunology, cancer, and metabolic disease pipelines, while prioritizing patient-centered clinical outcomes, for both growing market competitors and existing companies. To capture future development in the changing competitive landscape, not only will scientific brilliance be required, but also flexible, market-responsive tactics.
AbbVie. (2019, April 23). Abbvie expands immunology portfolio in the U.S. with FDA approval of SKYRIZITM(risankizumab-RZAA) for moderate to severe plaque psoriasis. prnewswire.com. https://www.prnewswire.com/news-releases/abbvie-expands-immunology-portfolio-in-the-us-with-fda-approval-of-skyrizi-risankizumab-rzaa-for-moderate-to-severe-plaque-psoriasis-300836934.html
AbbVie. (2024, June 18). U.S. FDA approves SKYRIZI® (risankizumab-rzaa) for ulcerative colitis, expanding AbbVie’s portfolio across inflammatory bowel disease. prnewswire.com. https://www.prnewswire.com/news-releases/us-fda-approves-skyrizi-risankizumab-rzaa-for-ulcerative-colitis-expanding-abbvies-portfolio-across-inflammatory-bowel-disease-302176163.html
Drugs.com. (n.d.). Dupixent (dupilumab) FDA approval history. https://www.drugs.com/history/dupixent.html
FDA. (2024, March 8). FDA approves first treatment to reduce risk of serious heart problems specifically in adults with obesity or overweight. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
FDA. (2024b, November 8). FDA approves new medication for chronic weight management. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management
Kimball, A. B., Prens, E. P., Passeron, T., Maverakis, E., Turchin, I., Beeck, S., Drogaris, L., Geng, Z., Zhan, T., Messina, I., & Bechara, F. G. (2023). Efficacy and safety of risankizumab for the treatment of hidradenitis suppurativa: A phase 2, randomized, placebo-controlled trial. Dermatology and Therapy, 13(5), 1099–1111. https://doi.org/10.1007/s13555-023-00913-3
Lilly. (2022, May 13). FDA approves Lilly’s mounjaroTM (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for the treatment of adults with type 2 diabetes. Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-mounjarotm-tirzepatide-injection-first-and
Liu, A. (2021, January 6). AbbVie’s post-humira life looks brighter with Skyrizi psoriatic arthritis win | fierce pharma. fiercepharma.com. https://www.fiercepharma.com/marketing/abbvie-s-post-humira-life-looks-brighter-skyrizi-psoriatic-arthritis-win
Merck. (2015, October 2). FDA approves Keytruda® (pembrolizumab) for the treatment of patients with metastatic non-small cell lung cancer whose tumors express PD-L1 with disease progression on or after platinum-containing chemotherapy. Merck.com. https://www.merck.com/news/fda-approves-keytruda-pembrolizumab-for-the-treatment-of-patients-with-metastatic-non-small-cell-lung-cancer-whose-tumors-express-pd-l1-with-disease-progression-on-or-after-platinum-containing/
Mikulic, M. (2025, March 13). Top pharmaceutical drugs by projected 2025 global sales. Statista. https://www.statista.com/statistics/973523/top-drugs-by-year-on-year-sales-increase/
Wojtara, M., Syeda, Y., Mozgała, N., & Mazumder, A. (2023, June 6). Examining off-label prescribing of Ozempic for weight-loss. Qeios. https://doi.org/10.32388/T6Y97S
Regeneron. (2025, June 20). Dupixent® (dupilumab) approved in the U.S. as the only targeted medicine to treat patients with bullous pemphigoid (BP). GlobeNewswire News Room. https://www.globenewswire.com/news-release/2025/06/20/3102521/0/en/Dupixent-dupilumab-Approved-in-the-U-S-as-the-Only-Targeted-Medicine-to-Treat-Patients-with-Bullous-Pemphigoid-BP.html
David Orchard-Webb, Ph.D., is a technical writer with broad interests including health & technology writing, plus extensive training and knowledge of biomedicine and microbiology. My Ph.D. and postdoc were in oncology and developing cancer medicines. I provide technical medical and other writing services for projects ranging from “knowledge automation” to pure pharma, to food safety, to the history of science, and everything in between. I also provide white papers, ebooks, meta-analysis reviews, editing, consulting, business, and market research-related activities in biomedicine, technology, and health. In addition to its well-known role in the development of medicines, I am a big believer in biotechnology’s ability to revolutionize industries such as food-tech, agtech, textiles & fashion.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


